|
|
FIFTY YEARS AGO, a graduate student named Stanley Miller tried to create life—or something like it—in a chemistry lab at the University of Chicago. To approximate the ocean of primitive Earth, he filled a glass bulb with water, methane, ammonia, and hydrogen, the chemicals that scientists speculated had dominated the early atmosphere. Then Miller hooked two electrodes to the bulb to simulate lightning, and flipped the switch.
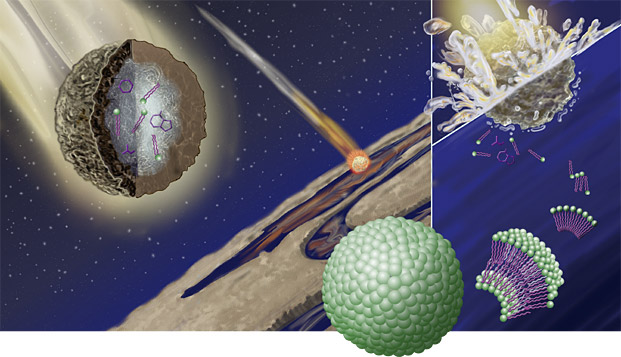
Miller let the mixture brew for a week. The “ocean” bubbled, converting to vapor that rose to the “atmosphere,” where a continuous current zapped the gas, imitating an electrical storm. Inside the glass bulb was a condensing column that worked like a cold soda can on a hot day: When vapor hit the column, the gas turned into liquid. Then it trickled back into the ocean to start the process again. While Miller didn’t find a growling monster bursting out of the flask at the end, what he did find was almost as exciting: The flask contained amino acids, vital components of every living thing. The implications were huge: If biological chemicals were so easy to make from scratch, many researchers believed that the other steps to generating life would soon fall into place. “It’s maybe hard for us to really appreciate how big the [experiment’s] impact was at the time,” says Max Bernstein, a chemist at the NASA Ames Research Center near San Jose, California. Miller and his colleagues “were seeing what we think of as the absolute basic components of life. So it seemed like it would be a short time before the origin of life would be understood.” Instead, 50 years later, Bernstein says, “In a way we know more—but we think we know less.” Researchers soon found that adenine, one of the bases of DNA, could form under similar conditions. And in 1969, a meteorite chock-full of amino acids fell near Murchison, Australia, hinting that some of life’s ingredients might have come from space. Yet try as they might, scientists have been unable to use these ingredients to create even the most lowly of organisms in the lab. Half a century of exploration has called into question Miller’s original premise that life began in the oceans, and alternative explanations remain unproven. The idea that the orchestrated complexity of life could have come out of random non-life is totally counterintuitive. “Most scientists find it too daunting a problem,” says Dave Deamer, a biochemist at the University of California in Santa Cruz. Though modern science can put a man on the moon and split the atom, the recipe for pond scum remains stubbornly elusive. The puzzle is made more intimidating by the fact that, with no physical record of the first organisms, there’s no way to definitively test theories of how they came about. At best, says Deamer, scientists will be able to set up model systems to show how life might have arisen. Bernstein and Deamer are among the scientists engaged in this quest. Rather than focusing on the planet’s early oceans for answers, they have both looked to outer space for clues. They are exploring the idea that we may owe our lives to crucial compounds that formed in tiny ice particles and rode down to the Earth on meteorites or space dust. The tallest hurdle to tracing the origin of life is that even the most basic form of self-sustaining life imaginable would have had to perform many tasks at the same time, such as harvesting energy from sunlight, chemicals, or heat, and using that energy to reproduce itself. It also must have had a genetic code to outline the basics of these processes, and a way of passing that code on to its descendants that allowed for some genetic change, but not too much. It must, in other words, have been able to evolve—otherwise life could never have gotten past that primitive first step. That’s a tall order for something that came together by chance, because it requires a melange of complex chemicals. The organic chemicals needed for life could not form from scratch in today’s oceans and ponds, because our atmosphere holds too much oxygen; oxygen reacts with organic molecules, ripping them apart before they have a chance of doing anything interesting. Fifty years ago, scientists conjectured that primitive Earth had an atmosphere rich in hydrogen rather than oxygen, one that would have favored the formation of long organic molecules, like amino acids, from carbon and hydrogen. However, geologists now think that although the Earth’s atmosphere contained less oxygen during the planet’s early existence than it does today, it still had too much oxygen and too little hydrogen to favor production of complex molecules. Thus, large amounts of organic molecules could not have formed in the ocean, as Miller thought, to form the rich “primordial soup” that he simulated in his experiment. SINCE 1953, a boggling cacophony of other hypotheses has arisen to explain life’s origins. Besides those who think we owe our lives to cosmic dust, some scientists subscribe to a modified version of the primordial soup theory, while others think life sprang from biochemical reactions at heat vents deep in the sea. Biochemist A.G. Cairns-Smith, of the University of Glasgow in Scotland, argues that the first genetic material might have been a self-replicating crystal. Gunter Wachtershauser, a German patent attorney who’s become a respected origin-of-life theorist in his spare time, postulates that life initially came about not in cells, but developed on the surfaces of special minerals that promoted crucial reactions. Francis Crick, one of the discoverers of the structure of DNA, even suggested that intelligent beings elsewhere might have “seeded” the Earth with extraterrestrial bacteria. Rather than crediting little green men with our existence, Bernstein has been studying a more prosaic breed of space invader: meteorites. On a recent afternoon at NASA Ames, Bernstein pauses in front of a display case of meteorites in the lobby of the building where he works. Most of them look like ordinary rocks; a dime-sized square of Martian rock mounted on a piece of wood resembles concrete. For Bernstein, meteorites are far more than museum curiosities. He is particularly interested in those known as carbonaceous chondrites, which bring organic chemicals with them to Earth. “When a meteorite falls to Earth, you can pick it up and analyze it, and be looking at perhaps a piece of the same asteroid or comet that fed the early Earth. These are things, literally, that are 4.5 billion years old, and you can hold them in your hand,” he says. By “fed,” he means that organic molecules from carbonaceous chondrites might have served as the starting materials for Earth’s first life forms. In his first-floor lab down the hall, Bernstein runs a deep-space version of Miller’s famous experiment. Instead of the relatively warm environment of the primordial soup experiments, he creates a cold, harsh vacuum simulating conditions in outer space to see what kinds of reactions might happen there. Common chemical sense dictates that very little will happen in a frigid environment (which is why food doesn’t spoil in the freezer). That’s because reactions require some energy to get started, usually from heat. Still, molecules frozen in ice crystals in space do get regularly zapped by strong ultraviolet and other radiation from stars—much more than what we get on Earth, where our atmosphere protects us. Such jolts might jar a molecule into reacting with its neighbor, prompting them to stick together, Bernstein says. This bigger new molecule may later bond with third molecule, and so on and so forth, until a complex molecule emerges. Such a process could take up to tens of millions of years in space, but Bernstein compresses the timescale into less than an hour in his studies. The first step is to look at telescope scans of the distant dust cloud whose conditions Bernstein wants to recreate. Different chemicals give off different colors of light, providing him clues to the gases that make up the dust cloud. He then allows a similar mixture of gases to slowly leak into the vacuum chamber, where they freeze onto an extremely cold surface. The temperature inside is about -445∞F, cold enough that the ice doesn’t even form crystals—just an amorphous blob. Then Bernstein bombards the frozen goo with UV radiation. After allowing time for reactions to take place, Bernstein analyzes the ice for substances such as the amino acids that make up proteins, and quinones, molecules that harvest energy in the cell—and has found that they’re there, though in vanishingly small amounts. Bernstein aims to find out where the organic molecules in carbonaceous chondrites come from. They may have formed in comets or meteorites with cores of ice, or even of water. Alternatively, the molecules could have been spawned billions of years ago in the fledgling solar system, when it was just a massive gas cloud infused with tiny grains of ice. To Bernstein, the difference between the two scenarios is crucial. It’s a question of universality: All solar systems condensed from interstellar gas clouds, so if biological chemicals formed within those gaseous mixtures, then “your starting materials are there in all the solar systems in the whole galaxy,” he says. But even if meteorites provided the seeds to the first complex biomolecules, how did those amino acids and quinones transform into life? Assuming that early life did build itself from space chemicals, then some scientists have simply traded distant dust clouds for warm oceans. The gap between non-life and life, after 50 years of conjecture and experiments, looms as large as ever. Enter Dave Deamer’s research. Deamer brings an unusual approach to the origin-of-life problem: Instead of focusing on how the inner workings of the cell fell into place, he’s working from the outside in—starting with the cell’s housing, components of which he believes might have come from meteorites. “Life as we know it began when it became cellular,” he says. In other words, the first organisms had to have a barrier separating themselves from the chaotic outer world. They needed skin, a membrane. With this idea in mind, Deamer decided to see whether membranes could spontaneously arise from the raw materials in meteorites—which isn’t as wacky as it seems. Certain molecules, detergents, have one end that likes to mingle with water and one end that, like grease, does not. When mixed with water, these detergents assemble into tiny spheres, lining themselves up into a double-layer barrier with their water-soluble ends facing out and their insoluble ends protected on the inside. These self-organizing molecules are the main components of cell membranes. Deamer theorized that if these kinds of spheres could form easily from extraterrestrial molecules that fell into water, that might be the kind of jump-start life needed. So he extracted organic molecules from a carbonaceous chondrite, added them to water—and found that spheres about the size of bacteria formed. Here was proof that the meteorite contained detergent molecules. Deamer found that the most prevalent detergent molecule in the meteorite was one called nononoic acid. His recipe for bacteria-sized membranes has the simple elegance of a middle school science fair project: Put one drop of nononoic acid in a drop of water on a slide, then add one drop fluorescent dye. A glimpse at the mixture under the microscope reveals round hollow spheres of different sizes, drifting around like bacterial ghosts. Under ultraviolet light, some of the spheres glow green because they contain the dye, while others loom darkly. The black spheres are intact membranes that have excluded the dye, Deamer explains, while the glowing spheres have slight flaws that allow some exchange with the environment. Such flaws would have been needed to allow the flow of nutrients into the very first cells, he believes. Using membranes as a starting point, Deamer is now trying to create an artificial cell in the lab. Scientists have long theorized that a molecule called RNA functioned in early cells as the carrier of genetic information before DNA evolved, so he did another experiment: He mixed building blocks of RNA—called nucleotides—with water, and found that freezing the solution could actually bring the pieces together in short chains. These chains could be coaxed to grow only as long as eight nucleotides each—a promising result, although the smallest useful RNA chains are at least 50 to 100 nucleotides long. Deamer concedes that RNA probably did not arise ab initio, but instead evolved from a simpler molecule. “There must have been some scaffolding, we call it, that provided a kind of sequence information for this, but we simply haven’t discovered it because it no longer exists,” he says. Deamer’s experiments have led him to question the supposition that life originated in the ocean. He has found that in saltwater, detergents form clumps, not spheres, so he theorizes that a freshwater pond would have been a more hospitable environment. And from his RNA experiment, he surmises that the pond where life originated would probably have been cold, not warm. As he sees it, meteorites could have fallen in or near a pond, bringing chemicals with them. Evaporation might have concentrated the chemicals, making them more likely to react with each other. Detergent spheres and short RNA strands would have formed separately, but over time, he thinks, a few RNA molecules would have been enclosed within some spheres by chance. And after many false starts, a stretch of RNA that could reproduce itself could have ended up inside a sphere. That would leave one more major hurdle to make Deamer’s hypothesis viable: To replicate itself, this promising sphere would have needed to produce its own detergent molecules. Fellow origin-of-life scientists aren’t holding their breath. Leslie Orgel, a Salk Institute biochemist, calls the idea that life’s building blocks came from space a “perfectly good theory,” but says, “at the moment there isn’t enough evidence to choose where molecules came from.” Similarly, evolutionary biologist Carl Woese of the University of Illinois says that no origin of life theory will convince him unless it produces a system of self-replicating chemical cycles, such as those that make energy or copy genetic material. “I don’t think we know enough to be sure of any of these suggestions,” he says. The origin of life is a fascinating problem, one that A.G. Cairns-Smith, the Scottish biochemist, has compared to a Sherlock Holmes story. But this greatest of scientific mysteries is far more complicated than any murder. Fifty years after Miller’s famous experiment, it’s impossible to say whether any of the detectives are on the right track. Judging by the current state of the origin-of-life field, it seems unlikely that the year 2053 will find children using high-tech chemistry sets to generate primitive organisms in ice cube trays in their freezers. Perhaps the origin-of-life field itself is stuck in a self-replicating cycle, a succession of red herrings and disappointments. Or maybe not. If nothing else, over the decades biologists have gained a much greater understanding of—and appreciation for—life’s awesome complexity. The double-helix structure of DNA was discovered in 1953, and just 50 years later, scientists have mapped the entire human genome and possess a basic understanding of how cells work. If life does not yield all its secrets in the coming decades, we can be sure that what we do learn will continue to astound us. |