There are strange people out there, says Jim Gimzewski. Since the UCLA chemist published a landmark study in the journal Science last year, he has received flasks of magic oil, religious books, and even a yellow-blue plastic structure the sender calls a "model of the universe." And a horror-movie producer asked him to contribute to the soundtrack.
That’s what happens when you claim you’ve heard yeast cells sing.
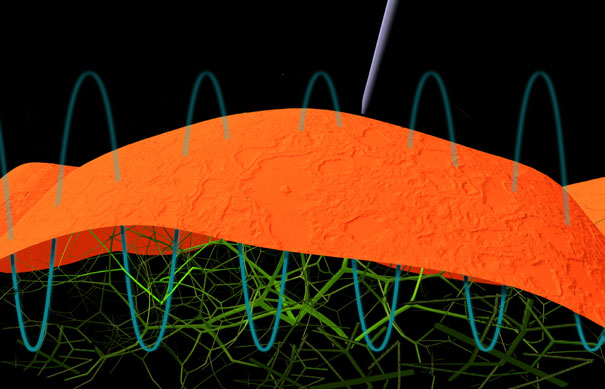
Gimzewski and his graduate student Andrew Pelling reported in August 2004 that they listened to something new and surprising: the sounds of ordinary yeast cells. Using the fine tip of an Atomic Force Microscope as a microphone, they found that the cells sing, making a constant sound at a frequency of about 880 Hertz, about the same as a telephone dial tone.
The finding launched an entirely new field Gimzewski calls "sonocytology." In the paper, his team suggested that the sound comes from tiny biological engines called motor molecules. The molecules transport components within all living cells and can also make cells shrink. "They are like a car engine you would listen to," he says. "I can think of no better direct, immediate, real time measurement of what goes on in a cell."
Indeed, the measurement of sound as cellular activity is so direct that Gimzewski now wants to use it to identify cancer cells. It’s known that the membranes of cancer cells are much softer than the membranes of normal cells. Gimzewski believes this difference will make them "sound" noticeably different from healthy cells. Listening to the sound of a few cells could be sufficient to diagnose cancer, whereas traditional methods need 10,000 cells to do that. This could one day enable doctors to diagnose cancer long before solid tumors develop. However, Gimzewski and his collegues have not yet demonstrated the feasibility of their idea — and any practical use for disease diagnosis could be many years away.
From buckyballs to cells
Long before he discovered cell sounds, Gimzewski made headlines twice in the 1990s while working at IBM’s famous research lab in Zürich. In 1996, he built the world’s smallest abacus, a manual computing device that uses movable counters, with buckyballs — hollow molecules made of 60 carbon atoms. In 1999, he made the cover of Science when he built the world’s smallest rotor out of a few atoms and showed that it actually moved.
With his uncombed grayish hair, Birkenstock sandals and a Blackberry cellular phone, Gimzewski looks like a futuristic rock musician as he sits in his office at UCLA’s department of chemistry and biochemistry. The 53-year-old practices Zen Buddhism and yoga in his spare time, writes poetry every day — some of it published on the web — and paints. One of his paintings on the wall shows a green man touching a hexagonally shaped structure. "This one is called ‘Man Touches the Atom,’" he says.
Art and science are very much related, he says, in that both are the result of intense human curiosity and imagination. But in high school, he recalls, "They wouldn’t let me do art and science. I had to do one or the other." He recently gave in to his artistic urge and collaborated with UCLA art professor Victoria Vesna on a "nano-art" exhibition at the Los Angeles County Museum of Art.
It was perhaps an open mind like this — and the terrorist attacks of September 11, 2001 — that explains why three years ago, Gimzewski was the first to listen to yeast cells. Everybody told him he was crazy. In fact, it almost didn’t happen. "The finding was the result of a whole series of accidents," he says.
The place where the amplified sound of yeast cells reached human ears for the first time ever is a small soundproof room in the basement of UCLA’s Young hall in Gimzewski’s lab. A six-foot-high mirror hangs at the end of the hallway that leads to the lab. Gimzewski and Vesna brought the mirror here from the nanoart exhibition at the LACMA to make the hallway look endless, Pelling says. Endless, that is, as seen by a webcam installed at the hallway’s other end.
The first room of the lab is filled with the desks of grad students and postdocs, and pictures of Buddhist mandalas hanging on the wall. Honeycomb-shaped, three feet wide boxes covered with transparent foil hang from the ceiling. The boxes are from an exhibit on cell sounds, where they served as video screens intended to look like cells of the body. The ceiling has also lots of pipes and encased lights of the kind one would see deep in the bowels of a ship. "Jim likes the industrial look," Pelling says. "He asked for these lights to look like warehouse lights."
And this lab looks industrial indeed. Two smaller rooms are filled with wires, oscilloscopes, and tools. One of them has a door with a soundproofing layer of foam on the inside. It harbors what Gimzewski and Pelling use to listen to cells: an atomic force microscope, or AFM. It’s surprisingly small, just a metal box a few inches on a side.
An AFM is like a very small record player, Pelling explains. It uses an extremely small tip made of silicon nitrite about 100 atoms wide -- 1/10,000th the diameter of a human hair. The tip sits at the end of a cantilevered arm and touches surfaces as it moves along to scan them. The arm bends every time the tip encounters a "hill" on its way across the surface. A laser beam reflects off the arm’s surface and changes its direction once the arm bends. Pelling measures the direction of that laser beam.
Usually, researchers use the AFM to create images of very small things such as cells. But Pelling and Gimzewski use it to "listen" to cells. To achieve this, all they do is leave the tip in place. They then record the vertical motions of the cell’s outer membrane or — in the case of yeast — the relatively thick cell wall.
Other researchers had used an AFM to listen to cells before. But these were cells that were known to move: beating heart cells. Nobody thought that non-beating cells would make any sounds at all.
Neither did Gimzewski or Pelling. Just like other researchers before them, they wanted to listen to heart muscle cells. The idea came up when Gimzewski was talking about science at a nanotechnology conference in Sardegna in 2001 with Carlo Ventura, a medical doctor from Rome. Ventura was studying stem cells that he wanted to implant into the heart region to help heal heart-attack patients. If you do that too early in the cells’ development, nothing will happen, Gimzewski says; too late, and the cells will be rejected. The cells have to be implanted at just the right moment — perhaps just when they start beating.
So Gimzewski told Ventura he would listen to the cells with his AFM to find the point in the stem cells’ development into heart cells when they start beating. Ventura sent him the cells in September 2001. But because of 9/11, they arrived three weeks late — and all the cells had died. U.S. customs thought the cells came from a terrorist lab that makes bioweapons, Gimzewski says.
But Gimzewski didn’t want to wait. He had prepared the AFM for the experiment, and he wanted to listen to cells, any cells. So he asked UCLA yeast biologist Joan Valentine to give him some of her yeast cells to calibrate the instrument with supposedly silent, non-moving cells.
Yeast cells are important organisms for biologists to study basic things about cells. They are ideal for genetic studies because they reproduce as rapidly as bacteria and have fewer genes than human cells. And much like plants, they have sturdy cell walls on top of their cell membranes. That’s why no one expected them to vibrate.
"I thought nothing would happen," Gimzewski recalls. "We just wanted to check the noise levels." Valentine’s group was even more skeptical: "They thought we were a bit crazy initially," he says.
Pelling had no expectations either. But when he first applied the AFM to the yeast cells, he saw oscillations on his screen. The pattern he saw was a sign not just of movement, but of regular movement. "I had no idea what to think," Pelling remembers. "I was completely shocked as I sat there looking at the screen."
When Gimzewski saw the pattern was regular, he got software from the Internet to convert it into sound. The result: A tone of about 880 Hz at room temperature.
But Gimzewski’s and Pelling’s initial excitement soon wore off when they started to wonder whether the sound could be an artifact, perhaps caused by something inside the microscope. They spent the next year to rule that out. "The first time we saw it I was very excited," Gimzewski says. "Then after that it was a long process."
The first hint that the sound emanated from the cells came when Gimzewski and Pelling soaked the cells in rubbing alcohol. That made the cells scream at first, Pelling says: The relatively pure tone of healthy cells gave way to high pitched, oscillating hisses, reminiscent of the background music in the shower scene in Alfred Hitchcock’s movie "Psycho." When the rubbing alcohol had finally killed the cells after 30 minutes, the sound was gone. This meant that the sound had to come from the cells. "I was at home analyzing my data and I almost fell off my chair," Pelling says of the moment he realized this.
But rubbing alcohol might affect the cell wall. So Pelling used sodium azide — a chemical known to kill cells without affecting the cell wall - and the sound went away as well. This proved that the sound had to come from inside the cells. Then Pelling poked the cells by pressing the AFM tip against them, and the sound changed. Finally, he increased the temperature to 30 degrees C. That almost doubled the pitch compared to the sound at room temperature. The scientists concluded the sound had to come from a biological process inside the cells.
"The temperature stuff I think was the real clincher," Pelling says, and Gimzewski agrees: "When we saw the temperature dependence, I was really over the moon."
Finding out what causes the sound
But even if the sound is real, what causes it? Gimzewski says it’s tiny motor molecules. They are like little molecular machines that move inside a cell to transport molecules and change the cell’s shape. Gimzewski calculated that the motor molecules need the same amout of energy to move that it takes to generate the sound. Moreover, azide — which makes the sound disappear — kills motor molecules. As final evidence, the motor molecules take about 1000 "steps" per second, similar to the frequency of the sound.
Gimzewski says it would probably take 100 motor molecules to make the vibration detectable by the AFM. An average car stereo system is ten thousand trillion times louder than one cell.
But there are skeptics. Kerry Bloom, a yeast biologist at the University of North Carolina, doesn’t believe 100 motors are enough. "I think there would have to be millions for them to hear it," he said. Instead, Bloom thinks ribosomes — complex protein factories inside cells -- may cause the sound, because one cell has millions of ribosomes instead of merely hundreds of motor molecules. Bloom says Gimzewski should test whether motor molecules are responsible for the sound by checking mutant cells that don’t have them.
Gimzewski is unswayed. "I can’t say it’s ridiculous, but apart from the fact there are lots of ribosomes in the cell — there are lots of other things in there as well," he says.
The cells of the human body must vibrate as well, Gimzewski says. "We know in every single cell in the body there are molecular motors, so on a nanoscale all these things go ‘dadingdadingdading,’" he says. Their combined power should make the sound audible — about as loud as the human voice. But even though we have trillions of cells, we don’t all make an 880 Hz thrumming sound when we walk down the street. The reason, Gimzewski explains while drawing wavy lines on a napkin in a Thai restaurant near UCLA, is that the waves cancel each other out, just like certain earphones use sound to dampen external noise. We never get to hear the cellular cacophony.
Another question is why healthy yeast cells oscillate at 880 Hz. To generate this sound, at least 100 motor molecules would have to move as a group — in lockstep. That’s possible, Gimzewski says, because motor molecules are connected to each other and to the cytoskeleton, a network of fibers that maintains the structural integrity of the cell. The cytoskeleton, in turn, is connected to the cell membrane, which is covered by the cell wall. These combined motions, he postulates, would make each cell wall pulsate like the membrane of a loudspeaker.
From yeast to human cells
Even though there are skeptics, Gimzewski says he doesn’t want to spend much more time trying to find out what causes the sounds in yeast. "My interest is to go into human cells," he says. "If we could do something useful for the benefit of people, that’s more interesting than spending my whole life with yeast cells." He has started a collaboration with Mike Teitell, a cancer researcher at UCLA, to listen to human cancer cells.
To do that, he explains, he needs a cantilever — the ultrathin silicon arm of the AFM that touches the cells — 50 to 100 times more flexible than the one he has used for yeast. "Human cells are much softer, so we have to make much softer cantilevers," he says. The reason is that the cantilever can’t be stiffer than the cell membrane; otherwise, it won’t respond to the membrane oscillations. A company in New York -- Albany Nanotech-- just finished making softer cantilevers. "We actually just got the cantilevers two days ago!" Pelling wrote in an e-mail on March 7. "I did have a chance to characterize them and they are more flexible; however, it will still be some time before we get to the cells."
Teitell says listening to cell sounds may be much more sensitive than current techniques to diagnose cancer, which mix cells from cancerous and healthy tissue, for example to check them for cancer-specific molecular markers. As a result, the few cells that could cause cancer might remain undetected. Analysis of cell sounds, in contrast, could identify these few cells. "We need to look at cells one by one so we don’t average out," Teitell says. "Say in a man’s cancer, 99.8 percent of the cells have one characteristic, and 0.2 percent have another characteristic. That 0.2 percent kills the patient because they escape the chemotherapy."
Listening to the vibrations of cells is a much better way to analyze cells than molecular markers, Teitell says, because it’s a more general way to look at a cell’s health. It’s known, for example, that cancer cells are softer than regular cells. Already, preliminary results show that different types of tumor cells cause different sound frequencies, he says. Unwilling to give more details because he is filing patents, Teitell says the goal is to make a library of cell sounds that researchers could one day use to diagnose cancer.
The cell sounds would change as the stiffness of the cell changes. But the day when AFMs could listen to cells in this way is at least a decade away, Teitell cautions. One problem is that to detect single cells, one would still have to remove the cells from inside the body using a biopsy. But in the far future, he says, it may be possible to build miniaturized AFMs that could detect the cells where they are, inside the body.
But some scientists aren’t convinced it will work. Ratnesh Lal, a research scientist at UC Santa Barbara, says that in addition to a change in frequency, the sound’s amplitude might go down in cancer cells. The motions might not be detectable anymore, even with an ultrasensitive probe.
Lal, who says he pioneered the AFM technique to listen to heart muscle cells in 1995, agrees that cancerous tissues show a change in stiffness. However, he says his own studies suggest the cell’s stiffness only changes locally, in certain areas of the cell membrane. This could make such changes hard to detect by an AFM. "Stiffness is not uniform," he says. "I don’t think there will be a major difference in stiffness between normal and cancer cells."
But new results that use the stiffness of cancer cells to distinguish them from normal cells give hope that Gimzewski might be on the right track. In April, Josef Käs and Jochen Guck of the University of Leipzig in Germany reported that they can measure the softness of cell membranes to diagnose cancer. They use a beam of infrared light to stretch cells. The more they can stretch, the softer the cells. The softer the cells, the more cancerous they are. They were able to correctly diagnose cancer after analyzing just 50 cells — as opposed to the 10,000-100,000 cells that are currently needed to diagnose cancer.
Pelling says the infrared beam method is impressive, adding that it differs from the AFM in that it measures the stiffness of whole cells. That could be an advantage, because it could average out local variations in stiffness, a possible problem with the AFM method, which measures oscillations of tiny parts of the cell membrane. It remains to be seen if UCSB’s Lal is right in that the AFM may pick up too many local variations and miss the big picture. "We just don’t know the answer yet," Pelling says.
But if the AFM method works, it may only take a single cell — not 50 — to diagnose cancer. It may even be possible one day to tell the cell type - say, a muscle or brain cell — from the way it sounds. And while the infrared beam method still requires isolating cells from the body, the miniature-AFMs Teitell envisions could enter the body to listen to cells where they are. All of that sounds promising, but even if it works, it will take many years until patients will have their cell sound checked in the hospital.
Using cell sounds to make music
While scientists are using cell sounds to find new ways to diagnose cancer, artists have become interested in the cell sounds as well. Pelling and UCLA art student Anne Niemetz used the cell sounds to create the first composition ever that utilizes cell sonics. The project was part of Niemetz’ MFA thesis, under the direction of art professor Victoria Vesna.
Niemetz says the piece used pure unprocessed cell sounds arranged in a 30-minute concert with five parts, moving from untouched cells to manipulated ones. "‘Observation to manipulation,’ we call it," she says. "It resembles the scientific method of discovery, where you first just look at something, until you manipulate your object of observation."
Beyond cancer research and art, Gimzewski believes the cell sounds may relate much more to real life experiences than we might think. "You meet somebody and say that person has good vibes," he says, "Why do you use the word vibration? That’s strange."
And the mail Gimzewski gets keeps getting stranger. Sometimes he even gets threats, he says: "Somebody emailed me and said my life was in danger because I had tapped into the cell vibration stuff and that the U.S. government would get me."
That sounds almost as threatening as the plot of the horror movie that uses
Gimzewski’s cell sounds. "The Curse of El Charro," directed
by Richard Ragsdale and slated for release soon, is about four college
girls in a remote desert town who are terrorized by the spirit of an evil
18th-century land baron. But Gimzewski is unlikely to ever watch — or
listen. "It’s an awful movie," he says. ![]()
Four clicks to the right, two clicks up
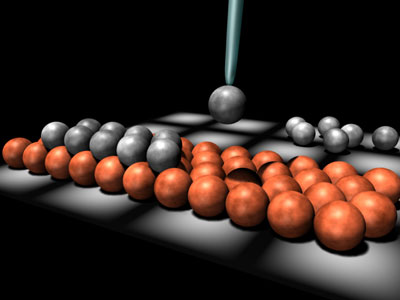
"Push to hear an atom," reads a blue box on Hari Manoharan’s web site. As I move the cursor over the box, I hear a sharp "click." The physicist at Stanford University uses the clicks not for fun, but to guide him while he moves single atoms of cobalt and other metals on extremely clean crystals of copper. To move, hear, and see single atoms, he uses an instrument called a Scanning Tunneling Microscope, or STM.
An STM has an extremely small metal tip, just one atom wide. The tip moves so close to the surface that the electron clouds between the two overlap. This can be measured as a current, just like the flow of electrons in a telephone wire is a current. This "tunnel" current changes as the distance between the tip and the surface changes. If the tip encounters a "hill" or "valley" on the surface, the distance between tip and surface can be kept constant by moving the tip up or down such that the current doesn’t change. Recording the tip’s position then generates a map of the surface.
But Manoharan uses the STM not just to visualize single atoms, but to move them around as well. That’s when the sound comes into play. To move single atoms, he has to use his "eye," the STM tip, as his hand. "The limitation is that we are effectively blind during this process," he says.
The tunnel current — amplified a billionfold — is Manoharan’s guide as he moves atoms. That current changes rapidly as atoms fall into spaces between the atoms in the crystal underneath it, because that changes their distance to the STM tip. Each atom is like a marble on an array of marbles, Manoharan says. "Imagine dragging one extra marble on top of this lattice — it’s going to click into the holes."
Each time this happens, there is a clicking sound. "Each click corresponds to when this atom clicks into a particular hollow in the lattice underneath," Manoharan explains. "You can listen to this atom and say OK, I am going to drag this thing four clicks to the right and two up, then it is in exactly that precise atomic location."
The type of click differs depending on what kind of atom he is moving, he says, because the weak chemical attraction between the atom and the tip changes.
Manoharan says he prefers listening to sound to looking at an oscilloscope
screen that shows the tunnel current as a wave. Our brain can process sound
information much more easily than visual information, he says: "Your
ear listening to these sounds gives you a lot more intuition as to
what’s going on."
![]()
ABOUT THE WRITER
 Andreas von Bubnoff
Andreas von Bubnoff
M.S. & Ph.D. (developmental biology), University of Freiburg
ABOUT THE ILLUSTRATOR
Melissa Thomas was born and raised in Sugar Land, Texas,
where she acquired a love for both science and art at an early age. At 18,
she mustered up the courage to fly across the country to study molecular
and
cell biology and art at UC Berkeley. After working for a few publications
and attaining some additional training in graphic design, Melissa was lucky
enough to study Science Illustration a UC Santa Cruz alongside 14 other
talented students. She interned with Scientific
American, and hopes to continue working in illustration and design for
science publications.