
The patient, a brown cat named Erik, lies motionless on his back as a nurse wheels him into the operating room. A caboose of medical equipment follows: heart monitor, anaesthetic machine, ventilator, and a network of wires and tubing.
"Alright, we're cutting," shouts one of the surgical nurses.
Hush overcomes the bustling operating room as a team of three surgeons, four nurses, and two anaesthesiologists snap to action. With a scalpel's quick slice, Erik's viscera splay out, protruding from his freshly shaved belly like a grisly fruit basket. In plain view are off-white intestines, a crimson spleen, and a bright magenta liver—not the dark purple you see in a butcher shop. There's little blood. The organs rise and fall with Erik's unhurried breaths. Two surgeons on opposite sides of the operating table huddle over the cat, their heads almost butting.
Waiting nearby, a sick cat named Teca—short for Tecumseh—will soon receive one of Erik's healthy kidneys.
In an increasingly common trend, cats like Teca are benefiting from top-notch health care as veterinarians borrow tools, techniques, and drugs from human medicine. But with the convergence of human and animal care comes escalating costs and tough decisions for owners. Teca's kidney transplant cost his owners more than $10,000. Others pay thousands more.
Since the mid-1980s, Clare Gregory, a veterinary surgeon and researcher at the University of California, Davis, has pioneered kidney transplants in pets. He's performed the operation in several hundred cats and 26 dogs. The transplants have been somewhat successful in cats—more than 90 percent survive the surgery and three-quarters live beyond a year. But with dogs, it's grimmer: A recent study showed just one in five lived more than a year after transplant. Slim chances, considering 95 percent of human kidney recipients live at least one year.
There's no single reason for the high rate of failure in these operations, but the drugs the animals take for the rest of their lives are the main cause, says Gregory. The medications stop the recipient's immune system from attacking the new organ. But they have nasty side effects like anemia and diabetes, and they make animals more vulnerable to cancer and infection.
Gregory is working to improve those odds. As director of the Comparative Transplantation Laboratory at UC Davis, he has spent more than 25 years developing and testing transplant rejection drugs for pets—and humans. Because the cost of producing new drugs is so high and the market for pets so small, human applications come first, he says. Only after pharmaceutical companies produce drugs commercially can Gregory adapt them to the pets he treats.
From humans to pets
"There are no diseases that occur in humans that we don't see in one or more animal species," says Niels Pedersen, a veterinarian at UC Davis who melds human and veterinary practices to offer better care to pets. He directs the Center for Companion Animal Health, a new $15 million clinic built with private donations. The center offers pets cutting-edge treatments that would never exist without human medicine. "There really is only one medicine," he says.
Comparative medicine—looking at the shared traits of human and animal disease—is not new. In ancient Greece, Hippocrates scorned veterinary medicine but studied animal pathology to gain insight into human diseases. The cattle plagues of 18th-century Europe spurred the first veterinary schools. By the late 19th century, France, Germany, and England boasted research institutes devoted to bridging the divide between animals and people.
From the start, surgeons studied organ transplantation in animals, too. Alexis Carrel performed the first kidney transplant in dogs in 1902 and later won a Nobel Prize for the surgical techniques he pioneered. But physicians quickly learned that successful transplants demanded more than surgical dexterity. Unless the kidney came from an identical sibling, the immune system attacked the perceived invader like an infection.
In the late 1970s, scientists discovered cyclosporine, a natural chemical made by a lowly soil fungus. It became the first blockbuster transplant drug. By stopping immune cells from dividing and attacking a transplanted organ, cyclosporine made long-term human and animal transplants feasible. Researchers performed some of the first tests on dogs. Soon Gregory started transplanting kidneys into beagles, a common lab breed. "It was nothing new," he says. "We just borrowed what had gone before."
By the mid 1980s, as kidney transplants grew more common in humans, Gregory became the first veterinarian to perform the surgery in pets. "We didn't start kidney transplantation in the clinic because we wanted to," he says. "We had so many clients asking for it." Twenty years later, more than a half-dozen veterinary hospitals around the country offer kidney transplants for pets. Cats are the most common recipients because their immune systems are simpler than those of dogs, and veterinarians must only match them according to one of two blood types. He performs about a dozen of the costly procedures each year.
Today's surgery is typical. Four months ago, Teca, a four-year-old Himalayan with penetrating white eyes, seemed perfectly healthy to his owners, Craig and Maera Busch. But during a routine check-up, they learned their cat was in late-stage kidney failure. Conventional therapies—medication, fluids delivered under the skin, and diet changes—failed, so the Buschs turned to Gregory. A week before the surgery, they flew to Davis from their home in Barrie, Ontario, an hour north of Toronto.
Surgery under the microscope
"Start the clock," Gregory orders. Erik's kidney sits in an ashtray-sized stainless steel basin resting on bloodstained surgical sponges, bathed in an ice-cold solution. Cut off from its blood supply and starved for oxygen, the kidney is slowly self-destructing. The surgical team has an hour to reconnect the organ to Teca, restoring blood flow.
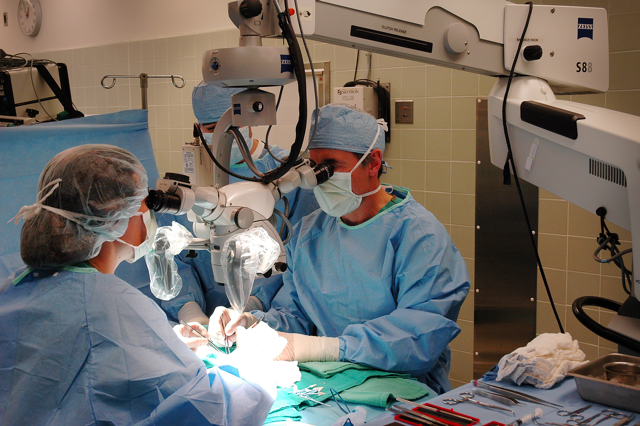
The artery, about the thickness of twine, is first. But even Gregory's experienced hands need help making the incisions and sewing the kidney in. A huge surgical microscope on a swing arm hovers over Teca's open abdomen.
A video camera positioned on top of the microscope beams the surgeons' view onto a television in the operating room. "It looks pretty," says a nurse, pointing to the monitor displaying Teca's open abdomen magnified 10 times. A puddle of saline the surgeons squirted into the cavity shimmers like a reflecting pool.
With his unblinking eyes never straying from the scope, Gregory makes a tiny cut into Teca's renal artery, which is clamped on both ends. Some blood trickles out for a second, then stops. The suturing begins. Working with tweezers and a needle threaded with silk suture so thin it's only visible under a microscope, Gregory and his colleague Margo Mehl tether the artery from the healthy kidney to Teca's artery with two knots on either end and sew the incision shut. The room is silent, except for Gregory's calm instructions.
A $30,000 cat named Princess
That cat kidney transplants exist is a testament to the demands of pet owners like the Buschs. "It didn't used to [involve] this much," says Maera Busch, waiting anxiously for Teca to get out of surgery. "When you had a problem, you pretty much put them to sleep."
No one knows how many cats and dogs suffer kidney failure each year; the ones who receive transplants are a tiny proportion. "There's a section of the population—not huge—that want the same thing for their pets as they want for themselves," says Gregory. But that decision isn't cheap. A feline kidney transplant can cost more than $10,000, and owners end up spending more on post-operative care, immunosuppressive drugs, and blood work. But veterinarians like Gregory say they are up-front about the costs. He has even urged people to reconsider the procedure if he thinks it's going to be a huge financial burden. "I don't want them to get a second mortgage on their house," he says.
But Deborah Casey, a 52-year-old middle school teacher from Albany, California, did exactly that. Several years ago, her cat Princess, one of four feral kittens she found living under a shed, developed kidney problems and failed to respond to conventional treatments. "I was so panicked I was going to lose her that I would have agreed to anything," she says. Casey estimates she's spent more than $30,000 caring for Princess, who got her transplant at UC Davis. Gregory is now one of the veterinarians overseeing Princess, who is doing well.
Like many owners of transplant recipients, Casey has no kids to support. She says her cats are like children, and she has no qualms spending money to care for them. Responses from friends and coworkers have been mixed; she only tells devoted pet owners about the transplant and the money she's spent.
"The knee-jerk reaction from some of the public is that these people are crazy," says Jonathan McAnulty, a veterinary surgeon who started the kidney transplant program at the University of Wisconsin. But he says they're devoted pet owners with disposable income. "I've been surprised at how uncharitable some people can be in condemning someone that would go this route," he says. "But they would never condemn someone who would buy a new car every three years."
Cowboy surgeries
When desperate pet owners approach Gregory for kidney transplants, he reminds them of the risks. "Don't get into transplantation if you don't think there's a possibility that your animal is going to die," he says. Nor is a new kidney a miracle tonic; rejection can happen at any time. "It's just a treatment, it's not a cure," he says.
For many pets and their owners, last-ditch surgeries like transplants are not the right choice, says Gregory. Medical technology can only do so much, and it often exposes the limitations of what veterinarians can do to save an animal's life. "The statement, 'Well it's going to die anyway' doesn't carry a lot of weight with me," he says. "There are ways to die, and I'd rather see an animal in comfort at home with good medical care than to have it die on my operating table." Gregory calls such doomed-to-fail procedures "cowboy surgeries."
In the operating room, the pace quickens. Gregory stitches the vein of the new kidney to a small incision in Teca's renal vein, completing the circuit of blood flow: oxygen in, carbon dioxide and waste out.
"Release the clamps," he orders.
Blood gushes into the newly connected organ, providing much-needed oxygen. "Mark the timer and put it in the record," says Gregory. A nurse notes the kidney was without blood for 54 minutes and 20 seconds. In past operations, the kidney has been disconnected for as long as two hours with good results, but the shorter the better, Gregory says.
The kidney is in, and the two other surgeons will close up the cat. Even in the operating room, there's time for a pun: "Your own little slice of heaven," Gregory announces to the team before scrubbing out.
A drug pipeline for pets
Though the operation went off without a hitch, the success of Teca's transplant is not assured. Twice a day for the rest of his life, the cat must take cyclosporine to stop his body from attacking Erik's donated kidney, but his compromised immune system will leave him vulnerable to disease. It's a balancing act between organ rejection and immune suppression, says Gregory. Over the next month, he will tweak Teca's medication to find the right dosage.
Current transplant drugs are indiscriminant. They act like a bomb, when a sniper would suffice. Cyclosporine haphazardly stops immune cells from dividing, the key to fighting a pathogen or keeping cancer at bay. It also has "10,000 side effects," says Mehl, Gregory's fellow surgeon. For this reason, Gregory is testing new antirejection drugs, not yet on the market.
Creating a new drug is a tortuous process that takes years and costs hundreds of millions of dollars. Along the way, pharmaceutical companies navigate several tiers of testing in animals and humans. Gregory partners with drug companies to do the preclinical testing required by the U.S. Food and Drug Administration. He tests transplant drugs on cats, dogs, rats, and primates at UC Davis to determine whether they're safe and effective. Only once a drug makes it through human trials and comes onto the market can veterinarians use it on pets. "We pretty much rely on the backbone structure of drug development for humans to allow us to have new drugs for animals," he says.
One example is leflunomide, a drug made by the Paris-based pharmaceutical company Sanofi-Aventis. The FDA approved it in 1999. Physicians use leflunomide to treat rheumatoid arthritis and occasionally to prevent transplant rejection. Gregory now includes it in a cocktail of drugs given to dog transplant recipients. As part of the development process in humans, he tested leflunomide on dogs in his lab, speeding its application to pets.
Unlike cyclosporine, the next generation of transplant rejection drugs being developed for humans work more specifically—like a sniper. Instead of tamping down the whole immune system, they go after specific molecular pathways. This focus leaves the body still able to fight infection and cancer, and causes fewer of the toxic side effects associated with cyclosporine. Many such medications are still in the early phases of testing in humans, but Gregory and other veterinarians are eager to add them to their repertoire. He's currently partnering with several pharmaceutical companies to help test the drugs, which he says could be available to pets within the next few years.
Ideally, transplant drugs and other pet medicines would be custom designed for animals, but that's unlikely given the minuscule number of animals that get organ transplants. In 2004, doctors performed more than 16,000 kidney transplants in humans—far more than any other solid organ—compared to the dozen or so kidney transplants that Gregory does each year.
One major difference between giving drugs to humans and to pets is the regulatory oversight. The FDA strictly controls human medications, while little analogue exists for animals. "In dog and cat medicine we're governed primarily by our own ethical codes on using drugs we think are safe," says Gregory.
The animal health-care boom
In 2006, Americans spent an estimated $36 billion on their pets. A good chunk of that—$9.4 billion—went to veterinary care. Fifteen years ago, people spent half as much on their pets. These figures reflect the convergence of human and veterinary medicine and the resulting increases in the cost of veterinary care, says Niels Pedersen, director of the new clinic at UC Davis that offers such top-tier care to pets. While Pedersen is an advocate of high-tech pet care, he says it represents a concerning trend.
Since World War II pets have gained status in the American home, as people migrated from rural areas and farms to sprawling suburbs. In many households, pets have become near equals to humans—even "child substitutes," says Pedersen. But he frets that the increasing cost of pet care, combined with the newfound social status of pets, could further complicate ownership and even obliterate the idea of "owning" an animal. "There's now a belief that if you can't afford veterinary care for your pet, you shouldn't have a pet," he says.
And as pets scamper up the social ladder, they gain new legal rights. Currently, animal malpractice suits, though on the rise, are awarded for the actual value of an animal—maybe a few thousand dollars. Pedersen expects this to change. "Nobody yet has won pain and suffering for $5 million," he says. "One of these days there will be some big settlement, and then veterinarians are going to realize that they've come the full way—they're now equivalent to M.D.s."
Teca is now out of surgery and recovering well. He'll spend a week in the hospital as Gregory watches for rejection and stabilizes his cyclosporine dosage. Two days after the transplant, Erik has fully recovered and is back with his new family, one $10,000 kidney lighter.
Is this veterinary medicine in the 21st century? Life-saving technologies such as kidney transplantation have opened the door to maladies of the human health care system: rising costs, malpractice, and ethical issues. While veterinarians like Gregory adapt human medical techniques and drugs to their animal clients, offering treatments that would never exist otherwise, the pet health-care system itself is adapting. Just be prepared to pay.
ABOUT THE WRITER
Ewen Callaway
B.A., biology, Colorado College
M.S., microbiology, University of Washington
Internship: Nature, Washington D.C.
 If bacteria could express joy (or any sentiment), they would be elated by
my departure from research. As I studied microbiology during the past five
years, countless microbes met their untimely deaths at my hands. In
college it was Xanthomonas, itself a killer of tomato plants. Salmonella
was my victim in graduate school, though I plead self-defense. Guilt
aside, there were other hints that research was not my ideal career.
Unlike my classmates, I spent nearly as long working on my prose as on
background reading. Volunteer work for a public radio science program
confirmed my suspicion that science writing might appeal to me. As I begin
my new career, I see my foray into microbiology as an experience that will
inform my writing. Still, I'm fortunate bacteria also are unable to hold a
grudge.
If bacteria could express joy (or any sentiment), they would be elated by
my departure from research. As I studied microbiology during the past five
years, countless microbes met their untimely deaths at my hands. In
college it was Xanthomonas, itself a killer of tomato plants. Salmonella
was my victim in graduate school, though I plead self-defense. Guilt
aside, there were other hints that research was not my ideal career.
Unlike my classmates, I spent nearly as long working on my prose as on
background reading. Volunteer work for a public radio science program
confirmed my suspicion that science writing might appeal to me. As I begin
my new career, I see my foray into microbiology as an experience that will
inform my writing. Still, I'm fortunate bacteria also are unable to hold a
grudge.
ABOUT THE ILLUSTRATOR
Jennifer Rodriguez
B.A., marine biology, Boston University
D.V.M. ,veterinary medicine, University of Wisconsin, Madison
 It's been a bit of a winding road so far, but no complaints, as everything
has started to fit together nicely. I grew up in Colorado, and went to
Boston University to study marine biology, traveling to Woods Hole,
Ecuador and Dominica during college. I worked at the Shedd Aquarium in
Chicago with marine mammals after college, and became interested in the
work of the veterinarians. Off I went to school again with the intent of
being an aquarium vet. It was three intense years in classes, and a great
final fourth year with actual patients. During that time, I started
drawing again and colleagues started asking me what I was doing being a
vet. So after some soul- and Web-searching, I found the UCSC Science
Illustration program. I will be returning to the Shedd Aquarium, this time
as a freelance exhibit artist, and also hoping to return to the vet school
to provide veterinary students and clinicians with better visual materials.
It's been a bit of a winding road so far, but no complaints, as everything
has started to fit together nicely. I grew up in Colorado, and went to
Boston University to study marine biology, traveling to Woods Hole,
Ecuador and Dominica during college. I worked at the Shedd Aquarium in
Chicago with marine mammals after college, and became interested in the
work of the veterinarians. Off I went to school again with the intent of
being an aquarium vet. It was three intense years in classes, and a great
final fourth year with actual patients. During that time, I started
drawing again and colleagues started asking me what I was doing being a
vet. So after some soul- and Web-searching, I found the UCSC Science
Illustration program. I will be returning to the Shedd Aquarium, this time
as a freelance exhibit artist, and also hoping to return to the vet school
to provide veterinary students and clinicians with better visual materials.
 Click to see a slideshow of the operation
Click to see a slideshow of the operation