MOST DIVERS WOULD HAVE IGNORED IT. The four-by-six-inch, cream-colored, jellylike mass clinging to an underwater cliff off the coast of the Philippines hardly even looked like an animal. Nevertheless, a team of American and Filipino scuba-diving researchers collected about half a pound of it, back in 1990. From this small store, they extracted less than a thimbleful of a chemical substance. Studies showed that it killed cancer cells in a test tube, but the scientists needed more of it to assess its potential as a drug in human patients. They returned to the Philippines several times, but it took eight years to find the animal again. |
||
A sea animal’s potent ingredient could provide a future medicine against cancer. But first, scientists must learn to make the chemical from scratch in the lab. |
In 1991, the researchers published their findings in a chemistry journal. That’s where Joe Konopelski, a scientist at the University of California, Santa Cruz, saw a drawing of the cancer killer’s complex structure for the first time. It fascinated him. He was inspired to open up his chemist’s bag of tricks. Konopelski’s repertoire includes many more chemical reactions than David Copperfield has illusions, but what he hoped to accomplish was no simple abracadabra. What the sea animal does biologically, Konopelski is attempting to do, chemically, in his laboratory. He’s trying to make the anticancer molecule from scratch. He’s not alone: A dozen labs around the world are working to synthesize the substance, called diazonamide A ("die-ah-ZONE-ah-mid"), which has "a structure like no one has seen before," Konopelski says. Even tiny quantities of it can kill cancer cells. "It will be a powerful biomedical agent," he predicts. Researchers have been racing to make diazonamide A for more than 10 years now, and while several labs have come close, not one has succeeded yet. Whoever pulls it off first could help save lives–and become quite wealthy. The means are as important as the end, however. Chemists tackling a specific challenge often invent entirely new chemistry. When Konopelski works on synthesizing a new molecule, he often finds that what he learns along the way can be useful for a broad range of problems, such as studying other pharmaceuticals or completely different compounds. Seeking medical cures from the natural world is nothing new. Humans had already been treating diseases with medicinal herbs for centuries when scientists first began to extract active ingredients from plants in the mid-1800s. This spurred some chemists to look for synthetic routes to manufacturing natural products. Researchers produced salicylic acid, a component of willow bark, from coal tar in 1859. Acetylsalicylic acid, a man-made cousin better known as aspirin, hit the market forty years later. Since then, the pharmaceutical industry has taken off. Plants as well as animals plucked from far-flung places, ranging from the rainforest to coral reefs, have yielded potential drugs. Isolating the compounds is often the easy part. Most natural products cannot simply be extracted, because plants and animals usually contain only tiny amounts of toxins that would make good drugs. For example, taxol, a drug used to treat breast and ovarian cancer, was extracted from the endangered Pacific yew tree’s bark in the 1970s. One patient’s treatment requires killing six trees, so using natural taxol would have quickly wiped out the species. Thus it was up to chemists to find a way to make the compound. In 1994, two research groups reported their successful chemical routes for synthesizing it. About 85 percent of all anticancer and antibacterial medicines are natural products or modified versions of them. For example, drug companies purify penicillin directly from mold. "No chemist can beat a bug at its own game," says Konopelski. Pharmaceutical firms also extract a compound similar to taxol from European yew tree needles, then convert it to taxol in a few chemical reactions. Sometimes, however, natural products are too toxic for human use, or too difficult to synthesize. Then, medicinal chemists may adapt them into similar compounds that can get the job done. In the hunt for natural remedies, chemists first looked for drug candidates from sources on land. A few decades ago, research groups met with indigenous peoples and learned how each group used local flora and fauna in healing. The scientists then extracted useful compounds from those plants and animals, and tried to make them in the lab. But now, many land sources are exhausted, so investigators are turning to the sea. "No marine natural product has ever been made into a drug," Konopelski says, "but the field is still in its infancy." Chemists who study marine natural products focus on organisms that appear defenseless and yet manage to escape predators. "We collect animals that we’re relatively confident will lead to something interesting. The hypothesis is that soft-bodied marine animals have developed chemicals for defense," says chemist Bill Fenical at the Scripps Institution of Oceanography in La Jolla, California. It was Fenical, along with collaborators from Silliman University in the Philippines, who in 1990 discovered the jellylike animal–called Diazona angulata–containing diazonamide A. Fenical has also identified another potential cancer drug, elutherobin, in a soft coral. Even though he includes only the most promising animals in his creature collection, Fenical doesn’t often find good drug candidates. "Over a ten-year period, diazonamide A and elutherobin are my two discoveries with true significance," he says. Diazona angulata actually contains two diazonamides, A and B. In 1990, Fenical’s group prepared a tiny crystal of a daughter molecule, called a derivative, of diazonamide B and sent it to Jon Clardy, a chemist at Cornell University in Ithaca, New York, for X-ray crystallography. In this technique, bombarding the crystal with X-rays reveals how the atoms are arranged within the molecule. For the technique to work, the crystal should be perfect or very nearly so. Neither diazonamide A nor B would form perfect crystals, so they couldn’t be directly analyzed themselves. But by determining the derivative’s structure, and then comparing it with data on the two diazonamides from other tests, Clardy and his colleagues were able to figure out their structures. At the same time, Fenical’s group started performing biological tests on the diazonamides. Of the more than 150 kinds of cancer cells available for study, Fenical chose to test the compounds against a certain type of colon cancer cell. "It’s traditionally one of the more difficult cancer cells to kill," he says. "If a compound inhibits its growth, chances are it will inhibit other cancer cells’ growth too." Diazonamide B showed mediocre cancer-fighting abilities, but miniscule amounts of diazonamide A killed the colon cancer cells. When divers returned to the Philippines to gather more of Diazona angulata, however, they found only its cousins. Lacking material, Fenical reluctantly turned to other projects. Finally, three years ago a researcher from the National Cancer Institute found the creature again in Philippine waters. The chemists extracted enough diazonamide A for more biological tests. Experiments revealed that, like many other cancer drugs, the substance prevents cells from multiplying by binding to tubulin, a molecule important in cell division and many other cell processes. However, the compound attaches to tubulin in a different place than other drugs do. "We realized that the molecule has a unique way of interacting at the cellular level," Fenical says. "We asked, Couldn’t this be part of a whole new set of anticancer drugs?"
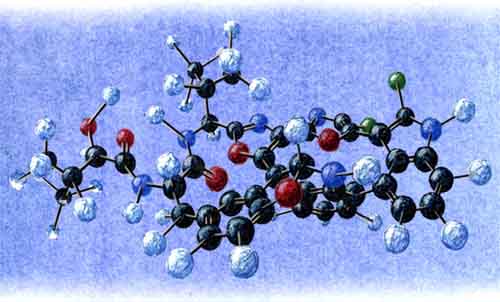
UNFORTUNATELY, WORK GROUND TO A HALT again when the new supply of material was almost gone. Making a commercial drug out of diazonamide A therefore rested upon finding a chemical way to copy it. The race to synthesize the precious molecule had already picked up steam in labs nationwide, after biological tests on the substance initially stopped in the early nineties. Joe Konopelski recalls his first encounter with the compound. "I was sitting there, at that desk, paging through a journal," he says, pointing across his office to a small table, "and I saw the structure. It was the coolest thing ever." After seeing diazonamide A’s structure, he tried to build the compound with a plastic molecular model kit that estimates how much space each atom takes up, and represents chemical bonds as overlaps connecting the atoms. Such "space-filling" models are more realistic than other types, but they aren’t perfect. "I couldn’t make the structure using these models. I couldn’t close all the bonds," Konopelski says. He decided he had to try to make the molecule in the lab. Diazonamide A contains several rings of carbon, nitrogen, and oxygen. It also includes two chlorine atoms–unusual in biological molecules–that lie near each other. In all, the compound contains 88 atoms. Konopelski’s task was to find a series of chemical reactions that would build the molecule, putting it together piece by piece. He began by working backwards. Looking over the structure of diazonamide A, he asked himself, "What is the last bond you want to make? Can you make it? Is it reasonable?" Once he made that choice, he next considered the second-to-last bond he would make. Then, the third-to-last bond. And so on, through the rest of the molecule, until he had synthesized the complete structure on paper. Another way to think of Konopelski’s task is to imagine that diazonamide A is the trunk of a tree. Above it, the tree has many branches that split many times into smaller ones. The tips of the smallest branches represent chemical "starting materials," pre-made compounds the chemist can buy. Each junction of two tree branches represents a possible reaction between compounds. Again, first working backwards on paper, the chemist climbs up the tree as he maps out a reverse route from the trunk (the final product) to the tree tips (the starting materials). Later, in the lab, the chemist climbs down the tree by performing in glass flasks a chain of reactions–beginning with the starting materials–that build the molecule step by step, branch by ever-larger branch, until he reaches the trunk and the final product is complete. However, the paper route is not a magic spell; often, parts don’t work. "Some pretty good people have walked up some blind alleys and had to step back," Konopelski says. Running into a dead end bothers some chemists more than others. Some believe if you can’t be first, be best, so they search for the most efficient route to the final product. Others think that the chemistry they learn along the way is more important than the final product, which they may never even make. They work at a slower pace, often taking detours to investigate interesting reactions that they discover. Konopelski definitely belongs to the latter camp, as does his graduate student, Brian Gerstenberger. "Once we learn one thing, it spawns 20 questions. That’s when you know you’re doing good science," Gerstenberger says. He sees himself as an architect, engineer, and construction worker wrapped into one. "I use paper to find how to build a molecule, then go out and do it," he says. The difference is that he is trying to build something he can’t see. A molecule of diazonamide A may be invisible to the naked eye, but compared to other compounds, synthesizing it is as complex a job as building a skyscraper. A closer look, however, reveals that the compound is partly constructed from smaller, simpler molecules. Called amino acids, they usually act as the building blocks of proteins. Diazonamide A isn’t a protein, but it contains fragments of several amino acids, which is extremely useful because these amino acids are commercially available. Twenty different amino acids are naturally found in proteins. All but one come in two forms that are mirror images of each other, known as left-handed and right-handed versions. Many molecules have left- and right-handed forms, and the difference between the two types can be essential. The most notorious example of this is thalidomide, a drug widely prescribed for morning sickness in Europe in the 1950s. Many babies were born with birth defects after their mothers took the drug. Further research showed that the therapeutic form of thalidomide, which relieves nausea, spontaneously converts in the human body to the harmful form that causes birth defects. At four points along the diazonamide A molecule, it has parts that can be either right- or left-handed. Three of these pieces can be purchased as amino acids. So Konopelski’s synthetic strategy has focused on making the fourth point, not commercially available, which has bonds linking it to four other carbon atoms–a rare and difficult arrangement to make. But difficult and complex chemistry is something that occurs all the time in Konopelski’s laboratory, a cluttered room on the third floor of the UCSC campus chemistry building. The lab smells faintly of acetone, the main ingredient in nail-polish remover. Stains on the floor bear witness to the years of work this room has seen. To protect themselves from dangerous chemicals, chemists do most of their work at a fume hood, an enclosed counter with an air intake vent overhead to draw away vapors. Glassware fills this hood: glass tubes packed with silica beads for separating mixtures, a still to remove water and oxygen from solvents, and two racks of test tubes with yellow or colorless liquid in them. The counter beside the hood is a mess. Small vials with white caps hold liquids and solids of different colors, labeled with numbers or names. Several flasks nestle in cork holders, and white powder sits on weighing paper. David Francois, a post-doctoral researcher in Konopelski’s lab, holds up a thin glass tube, called a pipet. A yellowish crystal the size of a sesame seed perches precariously on one end outside the tube. Under a magnifying glass, the crystal has a smooth face. The crystal is the product of a year’s work, but Francois isn’t sure he has made what he intended. His goal was to build an intermediate, a molecule partway between the starting materials and the final product–just one small branch en route to the diazonamide A tree trunk. Getting even this far was a challenge. Another researcher previously made almost the same intermediate compound, but it was an oil. Since X-ray crystallography requires a near-perfect crystal, Francois had hoped that he could produce a crystalline intermediate by beginning with a different starting material than his colleague–by using a left-handed, rather than right-handed, form of the material. However, the left-handed version wasn’t commercially available, so he had to make it himself. Francois spent a lot of time in the library, evaluating the approaches others had used and trying to determine the best one for his problem. "It’s like a puzzle," Francois says. "You study a lot of reactions; you have a target. It’s up to you to choose the best route to the target." Eventually he started testing different reactions, changing factors such as time and temperature to maximize the amount of product. After four months, Francois succeeded in building his left-handed starting material. And eight months later, he had his yellowish crystal of the diazonamide A intermediate. The next step is to prepare it for X-ray analysis, to see whether his creation is what he hopes it is.
REGARDLESS OF THE RESULTS, Francois knows his molecule has a problem. Recently, all labs working on diazonamide A suffered a major, shocking setback: Researchers learned they had been trying to make the wrong compound all along. Last December, biochemist Patrick Harran’s group at the University of Texas Southwestern Medical Center in Dallas announced it had successfully made the diazonamide A structure originally reported by Clardy in the early 1990s. Harran’s compound did kill cancer cells in the lab. However, it also degraded rapidly and behaved differently in other tests than did the natural product. So Harran double-checked the data Clardy had used to decipher the molecule’s structure. Harran’s group reinterpreted the results, publishing a corrected structure for diazonamide A that included three changes. This new structure is the true configuration of the compound extracted from Diazona angulata. The version everyone had been working toward is an interesting cousin of diazonamide A, but not a natural product. "I was surprised," says Francois. "But I was not disappointed. That’s research. Sometimes you have good news and sometimes bad news. Now the competition between groups starts again. It’s a whole new game." Still, the old game isn’t over yet. Harran’s group began attempting to make diazonamide A’s cousin four years ago. Of that time, the scientists spent a year and a half finding a way to create the last bond in their synthetic route. They eventually finished the job with a tricky reaction called photocyclization, in which a flash of light triggers a bond that completes a ring. "You very rarely see labs using photocyclization in the total synthesis of a large natural product because it’s hard to control," says Anthony Burgett, a graduate student in Harran’s lab. "We were winging it at the end." The photocyclization reaction in Harran’s method has about a relatively low yield, only about 35 percent of what they would ideally expect to produce. "For a photocyclization that’s not bad, but you’re throwing away two-thirds of a material that took you 20 to 30 steps to make," Burgett said. Those steps are expensive and an enormous time investment. Using Harran’s techniques, it takes about two months for one person to produce diazonamide A’s cousin. Jing Li, a post-doctoral fellow in Harran’s lab, is now repeating the method using more chemicals. In the end, Li hopes to produce 200 to 300 milligrams of the compound. (By comparison, one dose of the over-the-counter pain reliever ibuprofen is 200 milligrams.) That’s enough for additional studies of how the compound affects cells, but the researchers would need to develop a faster, more efficient way of synthesizing the cousin before it could become a serious drug candidate. With a feasible route for this cancer-killing cousin so close, is it worth it for researchers to continue working on the true diazonamide A? In Konopelski’s lab, for instance, the chemists are up a tree, so to speak. One of the original starting materials they used included an oxygen atom, but the revised diazonamide A structure contains a nitrogen in place of that oxygen instead. Therefore, to make the true compound, Konopelski and his colleagues must begin again from the branch tips. However, Harran’s lab has shown that diazonamide A’s cancer-fighting ability does not depend on that particular nitrogen atom. So, the Konopelski lab has decided to finish their work on the old structure while also beginning to tackle the new one.
Since Konopelski focuses on the chemistry he discovers en
route, and not just on the end product, he says it
doesn’t bother him that he must start his magic act
all over again. "Once you have the structure, someone
like me will make it," he says. "[It’s]
simpler now. It was hard to see before how the animal
synthesized it." With the true diazonamide A structure
in one hand, Konopelski is using the other to dig deeper
into his chemical bag of tricks. He’s sure the answer
lies in there somewhere. |
|