






Written by Kathleen
Wong
Illustrated by Anya Illes
There’s something about a hypodermic needle that makes even grownups cringe. A glimpse of the shining steel shaft with its wickedly sharp tip evokes a wave of unpleasant sensations: the pain of steel boring through skin and muscle; images of blood spurting red into a glass vial; and an uncomfortable realization of the fragility of the flesh.

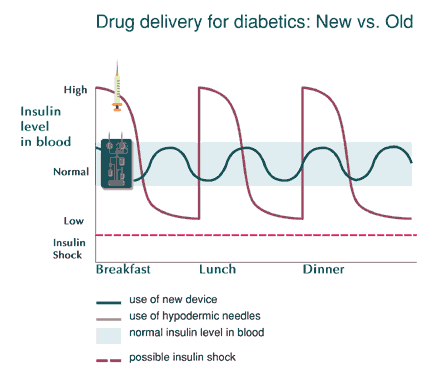
It’s a cycle of thought and feeling that nearly 5 million American diabetics experience more than once every day as they inject themselves with insulin to maintain the right amount of sugar in their blood. Insulin helps sugars move out of the bloodstream and into cells, keeping the blood at the right pH and cells well-fed. A malfunctioning pancreas unable to secrete enough insulin causes the type of diabetes that requires insulin shots.
Diabetics who need insulin shots usually treat themselves after meals to help their bodies process the blood sugar absorbed during digestion. With each injection, diabetics give themselves more insulin than they need at the moment so it will last until it’s time for the next injection. But these repeated, small overdoses of insulin take a heavy toll on the body over time. After many years, they can cause a host of circulatory problems leading to limb amputations, blindness, and kidney failure.
The only way to avoid small overdoses of insulin and other drugs is, of course, to supply a continuous flow of just the right amount of the drug. Traditionally, this has involved hooking up an intravenous needle to a pump the size of a shoebox—an unwieldy and impractical solution outside the hospital. Scientists have been working for years to develop better ways of delivering insulin and other medications. Recent innovations include a programmable, beeper-size pump hooked to a standard IV line, and foot-long inhalers that deliver doses of drug into the lungs.
Now, engineers at UC Berkeley are creating a portable and inexpensive drug delivery system capable of administering a constant supply of insulin and other medications in a convenient and painless manner. This tiny artificial pancreas mixes the drug in a silicon chip the size of a postage stamp, and injects it through a needle no larger than a mosquito’s stinger. The needle is so small that inserting it the necessary millimeter or two into the skin for the day or two of drug delivery doesn’t hurt. The whole system—including the wafer, a battery and enough insulin for 24 to 48 hours—has virtually no moving parts and fits inside a plastic shell no larger than a stack of eight credit cards.
“You could just slap it on your arm and hold it there with tape,” says Dorian Liepmann, the visionary bioengineering professor who has been leading the research since 1996.
Liepmann’s pressed oxford shirt and khakis look better suited to preppy Palo Alto than the laid-back People’s Republic of Berkeley, but they fit his ultra-organized, got-it-under-control attitude. Texts on fluid mechanics line his impeccable office. These books include one volume written by his father, Hans Liepmann, a physics professor at the California Institute of Technology. “He’s one of the grand old men of fluid mechanics,” Liepmann says proudly.
A desktop fountain fills Liepmann’s office, on the sixth floor of one of UC Berkeley’s concrete bunker buildings, with the sound of soft splashes. But rather than being relaxing, the sight of water spinning and swirling amid a cushion of bubbles seems to serve as a reminder of the fluid forces Liepmann strives to divine and direct.
Before Liepmann can send his little creation into the arms of a grateful populace, he must overcome several major technical problems, including leaky chip valves, shatter-prone needles and powdered drugs that won’t dissolve. “It’s going to be really cool,” says Liepmann, “if we can just make it work.”
Liepmann plans to achieve incredibly accurate control over the flow of fluids with his device to deliver such tiny, nontoxic doses of potent medications. Right now, the device can pump out liquids at less than a billionth of a liter per minute, or about the amount of ink needed to mark the period at the end of this sentence.
To control such small volumes of fluids, Liepmann uses a Lilliputian plumbing system made by standard microprocessor manufacturing techniques.
Engineers make computer chips in much the same way Mother Nature made the Grand Canyon—by depositing layers of different materials and then eroding them away. But instead of rivers cutting channels through formations of sand and minerals, a finely tuned sequence of acid and vapor baths eats through fine skins of the purest chemical compounds laid atop a silvery plate of silicon.
This process allows Liepmann to etch mazes of valves, mixers, channels and circuits onto silicon chips in any size or design he chooses. One experimental chip, made by one of Liepmann’s graduate students, spans only one centimeter on a side but contains five mixers, 72 valves and 35 pumps. The system’s small size makes for tough testing conditions; to watch his devices work, Liepmann must maneuver them under a microscope and display the action on a television screen.
A push of the on button starts the show. A highly efficient version of a watch battery powers up, opening a valve that is damming a reservoir of liquid insulin. The insulin is so concentrated that an entire one- or two-day dose can fit inside a pressurized pouch onboard the card. Designed by Becton Dickinson, the medical supply company helping to fund Liepmann’s research, the pouch squeezes the liquid insulin at a constant pressure into a channel on the silicon chip. Past the first valve, the insulin enters a mixer and is diluted with water. The diluted drug then flows out of the chip and into the needle, where it trickles into the patient’s skin.
The valve is a key component of the chip. Called a gate
valve, it operates like the slide bolt on a door. The gate
consists of a silicon block with a mass of only one
millionth of a gram, which dams a channel no wider than a
strand of human hair.
 When the battery is turned on, current
travels down platinum wires embedded into the silicon strata
of the chip. Like the coils in an electric blanket, the
wires line the floor of water-filled valve chambers
containing a cross-shaped block of silicon. The coils boil
water trapped inside the valve chamber. Bubbles from the
boiling water push on the cross’ arms and force it away from
the main channel. This holds the valve open. More bubbles
generated on the other side of the cross’s arms closes this
gate valve.
When the battery is turned on, current
travels down platinum wires embedded into the silicon strata
of the chip. Like the coils in an electric blanket, the
wires line the floor of water-filled valve chambers
containing a cross-shaped block of silicon. The coils boil
water trapped inside the valve chamber. Bubbles from the
boiling water push on the cross’ arms and force it away from
the main channel. This holds the valve open. More bubbles
generated on the other side of the cross’s arms closes this
gate valve.
To prevent the drug from flowing backward, one-way or check valves dot the channels between gate valves. The check valves consist of several silicon posts standing like sentries across the channel just behind a coil of platinum wires embedded into the passageway floor. Current flowing through the wires boils the flowing liquid (plain water or liquid drug) to create bubbles large enough to block the channel. Fluid flowing backward pushes the check valve bubble into the posts, where it must stop. Forward-flowing bubbles, facing no obstructions, travel far enough from the heating coil for the surrounding liquid to cool. The gas bubble recondenses into liquid at the lower temperatures.
Because both types of valves operate with thermal, or boiling bubbles, they pose several major problems Liepmann has yet to solve. The high temperature required to boil the liquid degrades heat-sensitive drugs. In addition, says Liepmann, the need to keep the valve fluids heated drains too much power from the batteries. “The heat’s being sucked away by the silicon all the time, and so you constantly have to put energy in to keep the valve closed,” he says. “That’s not a good situation.”
Nor has Leipmann been able to waterproof the gate valves holding back uncontrolled floods of drug and water. At boiling temperatures, the liquid on one side of a valve bubble tends to evaporate, travel through the bubble as a gas, and condense on the other side of the valve. The result is a net flow of liquid across the so-called seal. Valve leaks alter the amount of drug delivered to the patient, which may cause medical problems. The resourceful Liepmann says he’s now investigating the phenomenon as another method to control liquid flows through small spaces. And Burton Sage Jr. of Becton Dickinson believes that coating the gate valve surfaces with water-repellent materials could seal them.
To avoid the bubble troubles surrounding the thermal gate and check valves, Liepmann may substitute valves that make bubbles by splitting water molecules into hydrogen and oxygen gas with an electric current. He can form these electrolysis bubbles easily; it’s getting rid of them that’s the problem. “They usually don’t explode,” Liepmann says, “they implode. Very violently.” The bursts of energy would carve pits in the smooth walls of the channels and damage the seals on the valves. Liepmann thinks coating the bottom surface of the valves with a chemical catalyst could weaken the force of the implosion.
Mixing concentrated drugs with a dilution liquid before delivering them into the body is also proving problematic. Water and other liquids that seem thin and slippery when poured into a drinking glass act thick and viscous in tiny environments. The minute size of the channels and reservoirs makes liquids much more difficult to transport.
The culprit is surface tension, a peculiar force that makes fluids sticky. Surface tension lets water poured to the top of a cup rise above the lip and stick there a moment before running over. Surface tension keeps the water molecules in a raindrop together. But surface tension also makes liquids thick and difficult to mix in microscopic quarters. When the overall volume of liquid is small compared to the amount of liquid contacting the surfaces around it, as in Liepmann’s channels, the force of surface tension dominates the fluid’s behavior.
Imagine pouring cream into a cup of coffee. The thin liquids splash and swirl together, creating clouds and eddies like water in a whirlpool. This turbulence, a hallmark of liquids under little surface tension, makes mixing fast and effortless. Now imagine pouring honey into a cup of molasses. The two liquids are so thick that the turbulence of splashing can’t help them mix; the honey merely oozes atop the molasses in a uniform glob.
 Liepmann and his graduate students have devised a clever
way to mix fluids without the help of turbulence using a
mechanism that operates much like a jerry-rigged Jacuzzi.
The mixer consists of an oval chamber with pipes on each
long side that resemble the handles of an urn. Fluids enter
for mixing through a valve at one of the narrow ends of the
oval, and leave for delivery into the body through another
valve at the opposite end. Powered by nearby bubble pumps,
the urn handle pipes take turns sucking up fluid from one
corner of the oval and squirting it into another like
microscopic hot tub jets to circulate the fluid. The
researchers discovered that irregular siphoning and
squirting cycles thoroughly mix the most viscous fluids in
less than five seconds.
Liepmann and his graduate students have devised a clever
way to mix fluids without the help of turbulence using a
mechanism that operates much like a jerry-rigged Jacuzzi.
The mixer consists of an oval chamber with pipes on each
long side that resemble the handles of an urn. Fluids enter
for mixing through a valve at one of the narrow ends of the
oval, and leave for delivery into the body through another
valve at the opposite end. Powered by nearby bubble pumps,
the urn handle pipes take turns sucking up fluid from one
corner of the oval and squirting it into another like
microscopic hot tub jets to circulate the fluid. The
researchers discovered that irregular siphoning and
squirting cycles thoroughly mix the most viscous fluids in
less than five seconds.
Even though Liepmann’s drug delivery device still presents many technical problems, other scientists think it holds great potential as a drug delivery system. “The technology is very good for drug preparation, when you need to mix together components in exact quantities,” says Mauro Ferrari, a microfluidics engineering professor at Ohio State University in Columbus. “For example, if you’re mixing together blue and yellow dyes, you can be very careful about how much blue and yellow you mix to come out with exactly the right shade of green.”
However, some researchers have reservations about whether Liepmann can overcome problems posed by his tiny silicon-ceramic needles. Tejal Desai, a professor of bioengineering at the University of Illinois at Chicago, says the silicon could irritate the immune system. Desai says the body would grow a net of fibrous scar tissue around the needle, which would make it difficult for the drug to enter the bloodstream. But there are ways to overcome this problem, says Desai. “You can use silicon as a scaffold, but then make it more biofriendly by camouflaging it with biocompatible materials.” She says coating the needle with a gel called polyethylene glycol would reduce the likelihood of scarring and tissue damage.
The fact that the microneedles are much sharper than standard hypodermics, says Liepmann, will further limit the amount of scarring they cause.
According to Liepmann, the real health risk stems from his needles’ inability to bend. Instead of flexing like standard steel hypodermics, Liepmann’s glasslike needles tend to shatter if bent, leaving microscopic splinters in the skin. The body would probably immobilize the particles in capsules of scar tissue. The specter of microscopic slivers of silicon lingering within the skin of millions of diabetics could very well cause the Food and Drug Administration to reject the device.
Liepmann is already working to stabilize the needle shafts by molding them with internal support posts. He’s also investigating the feasibility of putting polymers on the outside to hold the pieces together if the needle breaks.
 Liepmann remains confident that he’ll find practical
solutions to these problems within the next two years. He
eventually plans to endow the device with a microprocessor
to control the valves and flow rates. Adding a computer
onboard would be easy, since microprocessors are made using
the same type of etching process Liepmann employs to
manufacture his drug delivery chips. With a smart drug
delivery device, he says, “you might have a little garage
door opener so that if you’re a diabetic and you’re eating a
sundae, you just hit a button and get a boost of
insulin.”
Liepmann remains confident that he’ll find practical
solutions to these problems within the next two years. He
eventually plans to endow the device with a microprocessor
to control the valves and flow rates. Adding a computer
onboard would be easy, since microprocessors are made using
the same type of etching process Liepmann employs to
manufacture his drug delivery chips. With a smart drug
delivery device, he says, “you might have a little garage
door opener so that if you’re a diabetic and you’re eating a
sundae, you just hit a button and get a boost of
insulin.”
As exciting as this sounds, Liepmann has even loftier goals for future models. “The Holy Grail of this whole thing is to also have a glucose sensor elsewhere in the body,” he says. The sensor would take continuous readings for glucose levels in the blood, and determine whether more or less insulin was needed to keep concentrations within the safe range. Using the body as an electrical conductor, the sensor would transmit an electrical signal to the pump and adjust its flow rate. “It’s not curing diabetes,” says Liepmann, “but we can control the system enough that we can have an external pancreas.” He and Becton Dickinson plan to make the drug delivery system inexpensive enough to discard after a single use.
Liepmann doesn’t plan to stop at revolutionizing insulin delivery. He’s already examining ways his system can improve the delivery of all kinds of drugs, especially those that are stable only in freeze-dried form. Such drugs spoil fast once they’re reconstituted into a liquid. Liepmann wants to make the device capable of reconstituting the drug after the battery is turned on, allowing the drug to stay freeze-dried until needed and prolonging the shelf life of the drug.
The U.S. military, which is funding part of the project, is taking a particular interest in this feature for treating soldiers in the battlefield. “The military wants to be able to put these in big cargo planes, fly them out and let them cook in the desert in Quonset huts for awhile, or let them get really humid and hot in a jungle environment, or freeze them in the Arctic,” says Liepmann.
Liepmann hasn’t yet worked out how to completely reconstitute a powdered drug with a dilution liquid. Mixing exact quantities of drug is critical—the wrong dose could poison or kill someone. At first, Liepmann’s team tried to make more than one batch of medication from a single large cake of dried drug. Each time the device needed more medication, team members reasoned, it could just flow more dilution liquid over the powder. “The first time, it works great,” says Liepmann. “But by the second time, it’s turned into cement.” He compares the effect to what happens when water runs over a pile of sugar crystals. The first few drops dissolve and carry away the surface grains of sugar nicely. But any residual moisture molecules will work their way into the pile of granules and bond the remaining crystals into a solid mass that is much more difficult to dissolve.
To deliver continuous doses of drugs that, once reconstituted, degrade within a few hours, Liepmann now plans to make cards with several chambers. Each chamber would hold one cake of dried drug. A microprocessor would tell the device when to dissolve the contents of each chamber as it is needed over time.
Abe Lee, who monitors Liepmann’s research for the military at the Defense Advanced Research Projects Agency in Arlington, says the technology will save medics time and expand their treatment options on the battlefield by allowing them to set up an intravenous drip in an active patient. He also says Liepmann’s device might someday provide fighting soldiers with lifesaving antidotes to chemical or biological weapons.
Adding narcotics to a card with an electronic controller could also help wounded soldiers and people with chronic pain keep their discomfort at more tolerable levels. “You can just push a button and it delivers the drug for patient-controlled pain relief,” says Becton Dickinson’s Burton Sage. And although the drug delivery device sounds like an easy way to get addicted to painkillers, it probably isn’t. Several studies have shown that patient-controlled pain relief not only reduces the amount of narcotic patients use, but it also helps them handle periodic pain surges without having to call for a doctor.
Sage says Becton Dickinson first plans to replace the despised hypodermic needle with the new microscopic version. He envisions coupling a standard syringe barrel to a centimeter-square pad of microneedles. The needle array would look and feel like sandpaper against the skin. This hybrid device would be able to inject liquid medications just as efficiently as a standard hypodermic, but without the pain.
Eventually, Sage says, the company plans to manufacture Liepmann’s entire drug delivery system. Although the University of California owns all the patents on the technology, the military and Becton Dickinson retain the right to manufacture the devices because they are funding the research. “The government can always march in,” Sage says, “but it’s not likely.” He says he’s confident the military won’t compete for the manufacturing rights because the government usually leaves mass production to private industry.
The device could also allow people to use other drugs previously dismissed as too toxic to take by mouth. The stomach absorbs most oral medications and sends the drugs directly to the liver. But some drugs, including several medications for Alzheimer’s disease, are extremely toxic to the liver if taken in pill form or added to the bloodstream in one large injection. Because Liepmann’s system delivers such small doses of drugs directly into the tissues, it might make many medications available that were shelved before.
Liepmann also envisions using the system to inject more than one type of drug at once. He can already manufacture the needles to contain more than one channel. Several autonomous drug delivery systems can fit on a single silicon chip because the pump systems are so small. “You can just use one needle and add the smarts to it with the electronics,” Liepmann says, “so you could have several different drugs, and then have the electronics control the delivery rate.”
The ability to inject multiple drugs with one system could drastically simplify the medication regimens of very sick people and also improve their health. “My wife, who is a geriatric psychiatrist, is very excited about this because her patients are often on several drugs,” Liepmann says. “It’s hard even for me to keep track of taking medications when I have to. When you’re talking about older people, and medications like cardiac medicine that are life-threatening, you can’t mess around.”
And if Liepmann has his way, people will no longer need to fuss with complicated drug regimens, the sick will be healed by wearing custom drug delivery cards, and only the very, very old will remember the visceral revulsion evoked by the sight of a hypodermic needle.
- BIOs
- WRITER Kathleen Wong
- B.A., biology and literature (double major), University of California, Santa Cruz.
Internship: U.S. News and World Report, Washington, D.C..
- ILLUSTRATOR Anya Illes
- B.A., biology, UCSC, 1995.
Internship: California Academy of Sciences; Great Wave (educational software).
Text © 1999 Kathleen Wong
Illustrations © 1999 Anya Illes