|
Better Living Through Hydrogel
Can synthetic “smart materials” that mimic the cell’s environment pave the way for stem-cell therapies? Janelle Weaver investigates. Illustrated by Angela Mele.
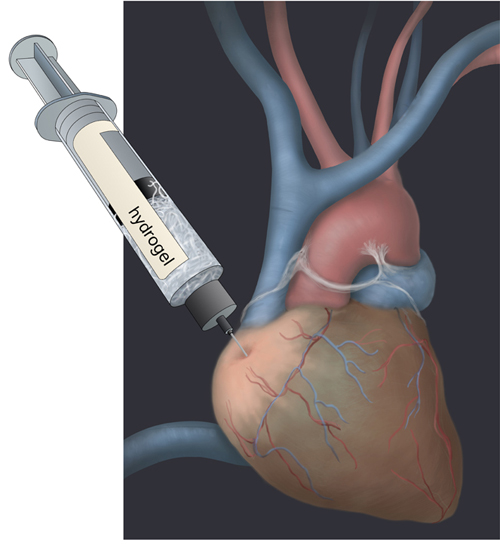
 Illustration: Angela Mele Illustration: Angela Mele
|
Kevin Healy is enchanted by medical plastics, right down to the props in his office. There’s the vascular stent, a Teflon-covered, man-made tube that keeps arteries open. Then there’s the Teflon-based vascular graft, a long tube that channels blood in patients with weakened vessels. These items are made of polymers: large molecules consisting of repeating units, which are relatively harmless inside the body. Healy wants to infuse them with stem cells, inject them, and repair damaged tissue in a wide range of organs, from hearts to brains.
A bioengineer and materials scientist at the University of California, Berkeley, Healy is developing networks of polymers, called hydrogels, which act as a type of jungle gym for stem cells to grow on. Once injected into the body, they promise to restore damaged tissue without causing an immune reaction. Made of the similar building blocks as cells and tissues, they’re naturally broken down by the body, leaving little trace.
“Think of a hydrogel as a contact lens. It’s dry—it’s just a large molecule—but when you add water to it, it actually holds the water like a sponge,” Healy says. A lab demo by graduate student Kimberly Kam makes that clear. When she injects a clear gel from a syringe into a flask of water, it looks stringy, like a piece of spaghetti. But when she pumps the same gel into a warmer body of water, it becomes stiff and opaque.
Indeed, the hydrogels are thermo-responsive smart materials that sense changes in the environment. If surgically injected as a liquid, they become stiffer when they warm to the body’s temperature. Then they’re ready to act as a scaffold for cells. And, by making the gel harder or softer, the scientists can coax stem cells to turn into different types of tissues.
Adding a concoction of growth hormones that stimulate human embryonic stem (hES) cells to turn into heart muscle cells produces a dish of heart cells that pulsate in synchrony. “Sometimes they beat so hard they tear off of the dish,” says Beth Irwin, a postdoctoral fellow in Healy’s lab. The cells grow on the hydrogel, and together they can be injected into damaged heart tissue to repair it. The goal is to bring this therapy to clinical trials in humans.
Much past research has focused on identifying the chemicals that shape a stem cell’s fate. But the importance of mechanical forces is just starting to garner attention. Healy is one of a handful of scientists around the world who is developing materials to guide what stem cells become. His lab is teaming up with chemical engineer David Schaffer and bioengineer Sanjay Kumar at UC Berkeley to pinpoint the molecules that sense mechanical properties, such as scaffold hardness, and translate these signals into a cell’s destiny.
Together, the three labs hope to set the stage for safer, more effective stem-cell therapies for a range of diseases, including heart disease, Parkinson’s, Alzheimer’s, retinal diseases, and spinal-cord injuries. But there’s a lot of tinkering to do, and they have yet to inject a patient with any of these wonder substances.
Healy’s hydrogels
The idea behind hydrogel is to mimic the normal environment found outside cells. Cells are arranged into tissues and organs by a sort of glue called the extracellular matrix (ECM), a network of proteins that anchor cells in place. Proteins hanging off of cells connect with the ECM, like kids’ arms on monkey bars. The hydrogel has little snippets of ECM glued onto it; these bind to the proteins hanging off the cells and hold the cells in place.
Once injected into the body, the hydrogel changes shape and lets cells grow into it. The hydrogel also releases chemicals that help cells recover. Eventually the artificial hydrogel is replaced by natural cells in the body. When injected into damaged tissue, hydrogels can stabilize and attract healthy cells, and sometimes help them to regenerate.
Sometimes the hydrogel carries stem cells, and other times drugs. The drugs have a targeted effect at the site of injection. “We’re putting the drug right at the site where the drug is needed,” Healy says. One example is atropine, which is used to treat myopia, or nearsightedness. The hydrogel’s structure helps hold the drug or drug delivery system in place. So far, the treatment has not been carried out in patients.
Healy’s lab is fine-tuning the three-dimensional design of the hydrogel to increase the odds that it will stimulate new growth in damaged tissue. Though his research hasn’t yet benefited humans, he aims to translate his basic research findings to clinical applications—but a host of challenges await.
Fickle stem cells
The endeavor to grow stem cells that are safe and effective for regenerative medicine had a setback in February 2009, when scientists reported the first known case of a stem-cell transplant that caused a tumor in a patient.
“Because they were stem cells, from one cell you can get a whole colony and that will essentially become a tumor,” Healy says. Human embryonic stem cells are a “clean slate” that can turn into any cell in the body, he says. They can form a malignant tumor if transplanted into patients.
The environment of the hES cells should be perfect to ensure they become the right cell type before being transplanted. “It’s basically the equivalent of making a cocktail and varying that cocktail,” Healy says. Over several weeks, cells are fed different chemical concoctions containing molecules called growth factors to direct them to the correct “differentiated” path toward a specific cell type, such as a heart cell or a neuron.
Hydrogels could help overcome problems with stem cells in two ways. First, they could be used alone, without stem cells, to help repair damaged tissue. This would bypass all the ethical, medical, and technological challenges associated with stem-cell research. For instance, Healy is now investigating whether hydrogels by themselves can strengthen eye tissue in lab animal models with myopia.
Second, hydrogels might help guide stem cells to the correct fate in a dish, thereby ensuring a pure colony of cells that would not later form a tumor inside a patient. Then the hydrogels and accompanying stem cells would be injected into damaged tissue. For this strategy to work, it’s essential to understand the chemical and mechanical cues that guide a stem cell’s fate.
Despite some progress, stem-cell research advances in fits and starts. It works one day, it works for two weeks, it works for two months, and then one day it stops. “You consider it a success when you can actually make differentiated stem cells in your lab, but it doesn’t always mean that someone else will be able to repeat it,” Healy says.
In contrast, hydrogels aren’t as touchy because they are completely synthetic. “Now, even though there’s this push for using stem cells, if you could get the job done without stem cells, it’s much easier from a practical and regulatory standpoint,” Healy says.
Another problem with stem cells is that they typically don’t survive and integrate with existing tissue once transplanted. In the case of cardiac therapies, “they sit there isolated by themselves,” Healy says. “They’re not electrically or mechanically coupled to the heart. They’re not doing anything. They actually could be doing more harm if they’re beating off cycle.”
He hopes hydrogels could help stem cells integrate with surrounding tissue by providing a scaffold that holds cells in place. Research in this area might make it possible to transplant stem cells into the heart so that they beat in synchrony with other heart cells.
Translating firmness to fate
In their Berkeley labs, Healy, David Schaffer and Sanjay Kumar are doing everything they can to get stem cells to behave. They hope one day to inject hydrogels filled with stem cells into patients with neurodegenerative diseases and spinal cord injuries. But first, they must figure out how stem cells choose their fates in a dish.
The first study showing that gel stiffness influences the fate of stem cells came in 2006 from Dennis Discher’s lab at the University of Pennsylvania. Then in 2008, Schaffer and Healy reported that neural stem cells respond to stiffness. Harder hydrogels are more likely to produce glial cells—cells in the brain that form tough scar tissue. Soft hydrogels more often lead to neurons. How the physical environment in a dish influences the fates of stem cells sheds new light on past experiments, which typically ignored the role of mechanical forces.
“As scientists, we forget that cells live in the body, not in a Petri dish,” says Theo Palmer, a non-collaborating neurologist at Stanford University. “It’s really difficult to say that all the work we do in the very constrained environment of the Petri dish really represents the range of cellular responses that occur in living, behaving organisms.”
The fact that stiffness affects stem cell fate may seem surprising to those outside the field, but there were previous clues in biology. One might think that cells are mechanically insulated from their environment, but even cells inside the brain pulsate as an aftereffect of a beating heart. Bones remodel in response to forces placed on muscles and ligaments. Scar tissue and tumors are harder than surrounding brain tissue. Even different cell types within a brain region have different levels of stiffness. Many cells are constantly pushing and pulling on their environment. “We think that that process of loading has something to do with what those cells will eventually become,” Kumar says.
Both chemical cues and stiffness interact to affect a stem cell’s choices. If you add a chemical that tells the stem cell to become a specific type of cell, making the matrix stiffer will tip the balance. If you add another chemical concoction that tells cells to replicate themselves, an intermediate stiffness will work best. The two kinds of signals interact to control a cell's behavior.
“Just figuring out how to do that by providing the appropriate chemical cues is something that the field is working out,” Kumar says. “To take the next step, to do this mechanically, is quite challenging but could potentially be very rewarding.”
While most past research has focused on how freely moving chemical cues can affect a stem cell's fate, Schaffer and Healy look at the role of molecules that are fixed onto the hydrogel matrix. The advantage of such unmoving cues is that they can present the cell with spatially organized patterns. These patterns cause receptors on the cells surface to cluster differently, which changes how cells respond to signals in their environment. Schaffer and Healy are now examining how the fixed patterns may help turn hES cells into neural cells.
By manipulating the physical environment of the stem cells, the scientists believe they will gain more control over determining their fate. That, in turn, will make it less likely for tumors to grow.
The future of hydrogels
Hydrogels show great promise because they are safe—most do not trigger immune reactions—and they can be easily mass produced. But this is not the case with stem cells. Up to a billion cells might be needed for a single patient. “That’s an enormous number of cells compared to what we typically deal with in the lab,” Healy says. Before stem-cell-laced biomaterials can reach the clinic, scientists must overcome several hurdles. “There’s no real clinical output yet,” Healy says. “I think we’re still far away from that.”
Another issue is directing the hydrogels to the damaged tissue once they’re injected. “It’s one thing to make a hydrogel in the lab, but how do you get it to the site where it’s needed? It would be nice to inject a liquid that hardens once it reaches the site of action,” Kumar says.
The first application may be injecting hydrogels alone, or with a drug, into diseased tissues to slow degeneration, says Theo Palmer of Stanford. Next would be to incorporate adult stem cells into hydrogels. “This therapy could be applied right away to treat stroke or spinal cord injuries,” Palmer says. “The lowest-hanging fruit is to deliver cells, not to restore the damaged circuitry, but to have them be healthier and happier after transplant and protect the local environment a little better.”
Ideally, scientists would like to use ES or induced pluripotent stem (iPS) cells, because they have the potential to become myriad cell types that could restore damaged nerve cells or tissue. That’s harder, because scientists will need to make sure all the stem cells become the right cell type. Any impurity can lead to a tumor. What’s more, the cells would have to integrate properly with the existing circuitry. “If you pattern the stem cells first and mix them with the right kind of scaffold, that would promote neurons to survive after transplantation,” Palmer says. “Then that becomes really exciting.”
The idea of using ES or iPS cells to treat diseases never would have occurred to Healy when he first began his scientific endeavors. As an undergraduate, he was captivated by artificial hearts, and his project involved fabricating vessels using plastic. He pointed out to his adviser that the blood kept clotting in the plastic replica; then Healy asked him why. His response: “Nobody knows how to make something that will work in the human body.”
By developing a gel that mimics the natural cellular environment, guides the fate of stem cells, and may repair damaged hearts, Healy has brought medical polymers a long way.
Story © 2010 by Janelle Weaver. For reproduction requests, contact the Science Communication Program office.
Top
Biographies
 Janelle Weaver Janelle Weaver
B.A. (psychology/neuroscience) Dartmouth College
Ph.D. (psychology/neuroscience) Stanford University
Internship: National Institute of General Medical Sciences, Bethesda
Like a bloodhound tracking an intriguing scent, I’ve always sniffed out scientific knowledge. I studied among the granite mountains of New Hampshire before gigantic magnets attracted me to Stanford, where I glimpsed nerve impulses dancing within human brains. Once life as a narrowly focused researcher set in, I yearned to experience more. I evaluated the latest neuroscience findings as an editor at PLoS Biology, but after rejecting 1,400 manuscripts and fielding fuming phone calls, I sought a profession that involved more positive interactions with scientists. I returned to Stanford as a writer in training, and was delighted to find that scientists graciously impart their wisdom away from the pressures of peer review. Now, on the inviting shores of Santa Cruz, I begin a lifelong quest, one that will perpetually put my nose to the test.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
 Angela Mele Angela Mele
B.F.A. (fine arts) Florida State University, Tallahassee
The summer after I graduated from Florida State, I discovered that the town I lived in was an untapped gold mine of edible mushrooms. Flowerlike chanterelles, brown felted wood ears, and luscious oyster mushrooms were in abundance in the woods and in my kitchen. I'm very attracted to abstract, amorphous shapes in nature, so drawing my specimens was an obvious next step. As I flipped through the pages of my mom's 1984 Peterson's Field Guide to Mushrooms, I realized that I would be very happy illustrating such books. I quickly determined that the Science Illustration Program at CSUMB was where I needed to go.
Top |

