|
| Illustration: José Miguel Mayo Hernández |
A hidden legacy of the Cold War is helping forensic scientists solve cold cases. Rina Shaikh-Lesko examines the evidence. Illustrated by José Miguel Mayo Hernández.
The squee-CHUNK, squee-CHUNK of the vacuum pump forces everyone in the cavernous room to yell. Physicist Bruce Buchholz peers at an aluminum container the size of a black bean. Inside its thick walls, a cavity as thin as a toothbrush bristle holds a tiny smudge of carbon in the form of graphite. It was culled from a tooth, extracted from a partial skull found in Canada four decades ago.
A hunter spied the skull in 1968 along the banks of the Nechako River near Vanderhoof, a small logging and farming village 500 miles north of Vancouver, British Columbia. A pit stop between Prince George and Fraser Lake, the village lies in the heart of the province’s mountainous back country. The skull had lingered in the vaults of a local museum until 2005, when the case was reopened in an attempt to identify the child using modern forensic methods.
Buchholz loads the tooth sample into a giant machine at Lawrence Livermore National Laboratories (LLNL) in California. The device, an accelerator mass spectrometer, measures the precise weights of atoms. It’s now a forensic tool, not just a research instrument; it can reveal when the tooth’s owner was born. The clue lies inside the tooth, in an unsettling imprint made by atomic bombs.
In the decades after World War II, generations of schoolchildren practiced “duck and cover” drills, scrambling under their desks against the grim possibility of nuclear attack. The silhouette of an expanding mushroom cloud came to embody our fear of annihilation by incineration. Little did we know that the bomb didn’t just scar our psyches. It left an indelible mark in our bodies as well.
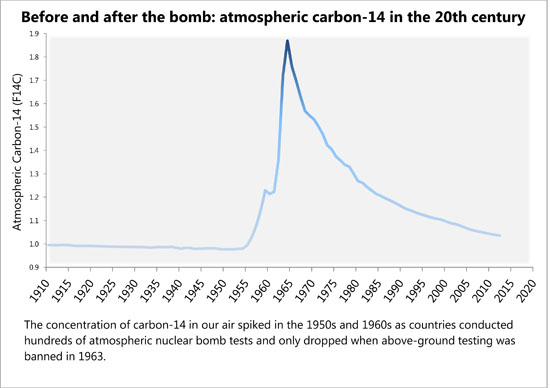
Hundreds of atmospheric bomb tests before 1963 doubled the amount of radioactive carbon in the air. It has decreased ever since, and scientists have tracked this fading echo. One residue of that pulse lies in our teeth. Enamel doesn’t regenerate once it forms, so the radioactive carbon used to make it is locked in for life, or longer — an internal calendar marking the years since the heyday of bomb tests. By measuring this carbon in a tooth, Buchholz can estimate when it grew to within 1.5 years.
For those born in the nuclear age, carbon-dating teeth from an unidentified body is the forensic equivalent of a runner’s stopwatch. That precision can narrow reams of possible missing-person matches down to a handful. Investigators can then refine the search with other techniques, such as DNA matching. The method, Buchholz believes, could help crack thousands of cold cases — if more investigators knew it existed.
A dark legacy’s silver lining
Manhattan Project scientists detonated the first nuclear bomb in mid-July 1945 in the desert of New Mexico. Less than a month later, United States warplanes dropped bombs on Hiroshima and Nagasaki, Japan.
After World War II ended, nuclear bomb testing intensified for nearly two decades as the Cold War deepened. In 1963, the U.S., the U.S.S.R. and the U.K. signed the Limited Test Ban Treaty, banning above-ground tests. The treaty slowed the pace of nuclear weapons development and curbed the toxic fallout that showered out of the sky.
 Graphics: Rina Shaikh-Lesko Graphics: Rina Shaikh-Lesko
|
By the time the treaty was signed, nuclear testing had doubled the amount of radioactive carbon-14 — a heavier form of carbon atoms than the more stable carbon-12 — in the atmosphere. Carbon-14 normally is created in trace amounts by cosmic rays walloping nitrogen atoms high in the atmosphere. Nuclear bomb explosions liberated millions of neutrons, creating carbon-14 that quickly joined with oxygen to make heavy carbon dioxide. Plants absorbed this, and it made its way into our food chain, our bodies and our teeth. “You literally are what you eat. The carbon from your food sources becomes part of you,” says Buchholz.
In the 1990s, scientists speculated they might read that signature for scientific purposes. “Everybody who’s been alive at this time has got this tracer in them, so let’s see what interesting science we can do with it,” Buchholz recalls thinking. One of his early projects at Livermore used bomb-pulse carbon to study how brain plaques form in Alzheimer’s disease (see sidebar: Reading the DNA Clock).
In 2002, postdoctoral researcher Kirsty Spalding of the Karolinska Institute in Sweden came across a write-up of Buchholz’s work. She thought she could apply the technique to her own research studying neural stems cells in adulthood, so she called Buchholz and asked for his help to test samples. She didn’t realize their collaboration would uncover a new forensic method as well.
Counting atoms, counting years
The accelerator mass spectrometer at LLNL, built in 1988, isn’t as energetic as the miles-wide accelerators used to shatter subatomic particles. But it’s plenty powerful for cutting-edge medical experiments. Two larger-than-life blow-up figures — an emperor penguin and Edvard Munch’s screamer — stand guard over it in a room the size of a basketball court.
When giving a tour of the noisy spectrometer, Buchholz sheds his measured tone and becomes animated. He recounts the studies he’s conducted on elephant tusks and heart cells. A scheduled cleaning will shut the spectrometer down for two days. As he describes using toothbrushes to clean gunk inside the accelerator, Buchholz can't suppress a grin.
 Photo: Rina Shaikh-Lesko Photo: Rina Shaikh-Lesko
|
Physicist Bruce Buchholz adjusts the accelerator mass spectrometer at Lawrence Livermore National Laboratory. |
|
|
The spectrometer's heart is a brown school-bus-sized tandem accelerator. A cesium gun shoots atoms at the smudge of graphite in the sample holder. The impact throws off electrically charged carbon ions, which are pushed and pulled down a 6-inch-wide tube by electric fields toward the accelerator. The ions zip through their 90-foot route at almost 3,000 miles per second. At full throttle, the roar is deafening. The sound, jokes Buchholz, sometimes seems to follow him on his 40-mile commute home.
The instrument, he says, “is just a big atom counter.” It actually counts isotopes: atoms of an element with slightly different weights, but the same chemical properties. Sensitive magnets steer the atoms through the tight route, pulling isotopes that are too heavy or too light off the speedway. Only the isotope of interest — carbon-14 in most biological studies — crosses the finish line. The machine measures the number of carbon-14 atoms to a few parts per quadrillion.
Spalding first learned of this instrument a decade ago when she wanted to know the ages of neurons. She suspected the radiocarbon in their DNA held the answer. Her new partnership with Buchholz gave her access to the Livermore instruments, but collecting human neurons is expensive. She had to find a substitute to test first — and it had to be from an animal that lived longer than the average lab rat.
She found her source an hour outside Stockholm, at a slaughterhouse where workers killed horses every other Tuesday. “I went to the local abattoir and had some of the worst and best moments of my scientific career,” she says with a short laugh. “They wanted to give me a horse’s head — like something from The Godfather — cut it off and give me a horse’s head.”
Spalding needed to validate her results by testing other horse tissues, so she settled on blood and teeth. Blood cells, replaced every few months, were a measure of the body’s youngest cells. Tooth enamel, formed near birth, was a marker for the oldest cells. She prepared the samples and mailed them to California for Buchholz to test.
Forensic possibilities
Weeks later, the possibility of using teeth to calculate the horse’s age dawned on Spalding. Curious, she tested human teeth. Early results were amazingly precise; she and Buchholz could estimate a person's age within a couple of years. That was much better than a forensic anthropologist’s best assessment, which has a 5-year to 10-year range.
She then approached Henrik Druid, chief forensic pathologist at the Karolinska Institute, with her idea. Tragically, they had a good test: bodies of Swedes recovered from the devastating tsunami that hit South Asia in December 2004. The bodies were battered beyond recognition, but Spalding’s method helped identify half a dozen Swedes. She and Buchholz published a paper in Nature outlining their findings. Soon, police investigators began asking for their help to identify bodies.
“It turned out that this dating is much more precise than anything being used in forensic science,” says Buchholz. “It seemed obvious, looking back on it, but no one had thought of doing this before.”
In the U.S. alone, an estimated 40,000 sets of unidentified human remains languish in evidence rooms. For bodies exposed to the elements for months or years, often little is left to analyze but a skeleton. Current forensic techniques, such as examining growth of teeth, can reveal the age of a young child to within a few months. But for adults, the range can be as wide as 20 years. “You could see how matching that to a missing person’s list is not a massive help,” says Spalding.
Bomb-pulse carbon dating cuts that range dramatically. Unlike DNA testing, it does not provide a positive match to a specific person. An accurate estimate of birth date, though, trims the field of possible matches from dizzyingly wide to manageably narrow.
“Man, that eliminates such a lot of persons from the list that you don’t have to worry about anymore,” says anthropologist Mark Skinner of Simon Fraser University in British Columbia. In 2005, Skinner directed the team that examined the 40-year-old skull from Vanderhoof.
A cold case thawed out
A forensic anthropologist had analyzed the skull when it was first found, estimating the child was seven to nine years old at death. Skinner’s team started from scratch, repeating the analysis with modern techniques such as testing DNA. They also examined plant matter stuck to the skull, hoping it might tell them where the skull had been. They extracted a dead leaf, a couple of leaf stems and bud scales from black cottonwood poplars. But that’s a common tree in the woods around Vanderhoof, so it didn’t reveal much.
One of Skinner’s graduate students mentioned Spalding and Buchholz’s work. The lower jaw wasn’t recovered, and only one of the child’s erupted baby teeth clung to the upper jaw. He sent it, along with an unerupted molar buried in the upper jawbone. Skinner told them nothing about the case to avoid biasing their findings. Buchholz and Spalding estimated the boy was probably born between 1959 and 1961 — just a few years before the world edged as close as it ever would to nuclear war, and atmospheric bomb testing peaked.
Skinner was impressed by the method's accuracy. “You’re getting the age of that enamel, plus or minus one year. That’s brilliant,” he says. “If the death has occurred in the last 50 to 60 years, I would use bomb carbon every time, rather than my own guesstimate based on how much I think the bones have changed since death.”
Others, like anthropologist Alison Galloway of UC Santa Cruz, aren’t sure bomb-pulse dating will be broadly useful. Now the campus provost, Galloway does agree that some cases might benefit from the approach, such as victims of a serial killer. “If you’re looking at a grave where there is no idea of the identification and no idea of the age, having this would be extremely helpful,” she says.
Former FBI agent Thomas Jourdan saw first-hand how bomb-pulse carbon dating could help solve cases when he was stationed at LLNL. Now at the University of Central Oklahoma’s Forensic Science Institute, he teaches the next generation of forensic scientists. “This isn’t something applied to every case,” he says. “It is a bit esoteric and it does come with limitations. But for certain things, it’s kind of magical.”
A family’s closure
When Skinner’s colleagues examined the skull, they concluded it belonged to a white child. DNA analysis revealed it was a boy. They also estimated he was four years old — half the age suggested by the examiner in 1968. Buchholz and Spalding’s radiocarbon dating yielded the same age estimate — and pointed to a possible match.
It was enough for British Columbia authorities to approach a Vanderhoof family whose young son had gone missing in 1965. Although the scientists declined to provide identifying information for this story, a search of public records revealed the details.
 On March 25, 1965, a pair of young friends, Robbie and Karen, went out to play around lunch. Half an hour later, Karen returned home alone. A search for the boy turned up nothing but a hole in the ice at the edge of the Nechako River, near the mouth of Stoney Creek. On March 25, 1965, a pair of young friends, Robbie and Karen, went out to play around lunch. Half an hour later, Karen returned home alone. A search for the boy turned up nothing but a hole in the ice at the edge of the Nechako River, near the mouth of Stoney Creek.
A week later, the local newspaper carried the news: “Karen's overalls were wet and it was understood that she had tried to pull her little friend from the water. It runs swift at that point, and was about five feet deep.” The story noted the body hadn’t been found. “Vanderhoof residents were shocked to learn that the Nechako River had once again claimed the life of one of our young citizens,” the reporter wrote.
The mother of the missing boy had died a few years before Buchholz and Spalding tested his teeth. Skinner’s team compared DNA from his father and oldest sister to DNA extracted from the skull. The match to the dad was shaky, but the match to his oldest sister — along with other forensic evidence — was strong enough to officially identify him. A death certificate was issued and the remains returned to the family, 44 years after Robbie went missing.
“It was so far back that I was surprised to hear anything. I didn’t think we’d ever find him,” says Frank McDougall, Robbie's father. The family interred Robbie's ashes in a crypt, next to his mother's urn.
“It’s satisfying to solve a case that’s been open for a very long time,” says Buchholz. “It’s nice to give families closure.”
Buchholz now gets a request to analyze tissue for forensic cases about every six months. He could handle more, but bomb-pulse dating isn’t widely known. It could take years for news of the technique to trickle out to forensic investigators. To speed that process, Spalding spoke at a forensic conference in Newfoundland in September 2012. She’s already seen an uptick in the number of requests from investigators.
Thousands of missing-persons cases moldering in police cabinets could benefit from the stamp of the atomic age we all carry in the skin of our teeth. Despite the terror it bred, the bomb — as one family in Canada already has learned — has the power to end decades of painful doubt.
Sidebar: Reading the DNA Clock
 Forensic applications are just one way to use the surge of radioactive carbon created by nuclear bomb testing. Initially, Bruce Buchholz and Kirsty Spalding used bomb-pulse dating of DNA to estimate the age of neurons. “The DNA, in a way, acts as a time capsule,” says Spalding. Other parts of the cell, such as proteins and lipids, are replaced regularly. But when the cell makes its DNA, it keeps it for its entire lifetime. “So if you can work out how old the DNA is, you can work out how old the cell is,” she says. Forensic applications are just one way to use the surge of radioactive carbon created by nuclear bomb testing. Initially, Bruce Buchholz and Kirsty Spalding used bomb-pulse dating of DNA to estimate the age of neurons. “The DNA, in a way, acts as a time capsule,” says Spalding. Other parts of the cell, such as proteins and lipids, are replaced regularly. But when the cell makes its DNA, it keeps it for its entire lifetime. “So if you can work out how old the DNA is, you can work out how old the cell is,” she says.
They applied a similar technique to look at cell turnover in heart cells and fat cells. Surprisingly, heart cells can regenerate during a person’s lifetime, while fat cells in obese people turn over more slowly than in normal-weight people, they found.
Buchholz has used bomb-pulse carbon dating in other high-profile arenas. For the FBI investigation of the anthrax mailings in 2001, he concluded that the spores were only a few years old and probably did not come from a known international stockpile. He has estimated the age of ivory tusks — which sell for $900 per pound on the black market — well enough to detect when a tusk is from a recently poached elephant.
Buchholz is now using the technique to study cell turnover in cancer tumors. If he can show when and where the cell growth mechanism goes berserk, researchers might have new targets for drugs. His challenge now is finding large tumors that yield enough DNA carbon. Each cell contains only a minuscule amount of DNA, and the accelerator mass spectrometer requires at least 50,000 to 100,000 cells’ worth. It’s slow work, but the prospect of unlocking a mystery of cancer cell growth pushes Buchholz onward.
Story ©2013 by Rina Shaikh-Lesko. For reproduction requests, contact the Science Communication Program office.
Top
Biographies
 Rina Shaikh-Lesko Rina Shaikh-Lesko
B.S. (biology) UC Riverside
M.P.H. (epidemiology/biostatistics) San Diego State University
Internship: California Institute for Regenerative Medicine (San Francisco)
My first reporting gig was with my junior high school newspaper. Soon I was more interested in dissecting frogs, so my reporting career went on hiatus for a couple of decades. Instead, I became an epidemiologist.
The challenge of epidemiology is what drew me. How do we prevent illness in large groups? I came to admire the tenacious microbes I was trying to eradicate: measles, hepatitis, influenza, pertussis, and other vaccine-preventable diseases. But over time, I grew restless with long stretches of poring over dry data files.
I got more out of the stories behind the science: the globe-trotting disease detectives who uncover novel viruses, the troubling deaths from drug-resistant bugs we thought we’d beaten. I want to share those stories, so I'm dusting off my reporter's cap—though it'll need to stretch a bit.
Rina Shaikh-Lesko web site
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
 José Miguel Mayo Hernández José Miguel Mayo Hernández
Internships: National Geographic, Life Lab (Santa Cruz)
I have drawn all my life; it's innate, natural, and almost automatic. If I have a pen in my hand it's impossible not to be scribbling something. I received most of my artistic training during my childhood, in the School of Fine Arts of St. Eloy in Salamanca, Spain. Later, during my time at University, my love for nature, botany and entomology led me to study forest engineering. I completed my engineering studies in Spain at the University of Valladolid and at the University of Santiago de Compostela, and pursued further training in Portugal at the Instituto Politécnico de Coimbra and also at the University of Costa Rica. I independently developed my interest in science illustration, learning both traditional and digital design techniques in order to pursue a career in this mix of art and science.
José Miguel Mayo Hernández web site
Top |