|
Illustration: Maayan Harel |
| Nicaraguan communities unite to halt a deadly virus while scientists decipher its patterns of infection. Beth Marie Mole reports. Illustrated by Maayan Harel and Nicole Wong. |
 |
All it takes is one tiny puddle. Madgalena Zamora raps another rickety door, sweating in Managua’s 100°F weather. Along the dusty roads of Barrio Jose Benito, she goes house-to-house, inspecting neglected vases, patio water barrels, and collected drips under sinks, searching for them: twitching jelly larvae. If left alone, they could transform into mosquitoes carrying the deadly dengue virus. Government fumigation and pamphlets have failed to combat the plague. But Zamora and dozens of other brigadistas that buzz around Nicaragua’s capital have triumphed, slapping down dengue infections by 60 percent. It’s rare good news amid mysterious spikes in severe disease and a global dengue boom.
“What got me involved was the data,” says Zamora. “Our homes were positive. My son was positive.”
The data comes from another unlikely source: Eva Harris, a dancing American scientist who came to Nicaragua in the 1980s—before dengue was a household term—to help in any way she could. Since 2004, the UC Berkeley epidemiologist has been following enigmatic outbreaks of severe dengue diseases in children.
 Of those infected with dengue, most get mild dengue fever—like seasonal flu—but others plunge into shock from internal hemorrhaging. In critical cases, death can come in less than 48 hours. Fifty years ago, a handful of dengue cases haunted only a few countries, mostly in Asia. Nicaragua wasn’t affected. Today, dengue is endemic there and in more than 100 other countries, causing up to 100 million cases per year. It’s the most common mosquito-delivered plague on the planet, beating West Nile virus and malaria. Of those infected with dengue, most get mild dengue fever—like seasonal flu—but others plunge into shock from internal hemorrhaging. In critical cases, death can come in less than 48 hours. Fifty years ago, a handful of dengue cases haunted only a few countries, mostly in Asia. Nicaragua wasn’t affected. Today, dengue is endemic there and in more than 100 other countries, causing up to 100 million cases per year. It’s the most common mosquito-delivered plague on the planet, beating West Nile virus and malaria.
Despite community campaigns around Managua, more and more kids have sardined into medical centers suffering from fever, rapid heart rate, and cold hands and feet in the sweltering summers. At Managua Children’s Hospital, where they’ve just installed an electric fan in the intensive care unit, Harris unfurled what is now an archetypal defense of coupled community efforts and clinical detective work. Her tenacious crusade unveiled how the disease boils up to deadly peaks.
But dengue is still on the move. Warming climates have made new territories cozy for mosquitoes, and dense, shoddy urban sprawls provide biting banquets. One of the mosquitoes that can carry dengue has now invaded the southeastern U.S. Without a clear understanding of the global disease level, epidemiologists can only guess where dengue and its burden will strike next.
To catch a virus
Researchers at the U.S. Centers for Disease Control and Prevention estimate that between 100 and 800 years ago, dengue jumped from monkeys to humans in Asia. It made the leap four times, creating four types of dengue virus that now co-circulate through populations around the world—including in Nicaragua.
Dengue struck there in the 1980s, after an earthquake in 1972 decimated Managua. The political revolution that followed sunk the country’s economy, and shoddy urban development sprawled from the crumbled city. Electricity is still hard to come by, and water only runs out of the tap a few hours each day. Managuans relegated their water management to storage barrels, such as the ones Zamora inspects on neighbors’ patios. Dengue’s hosts, Aedes aegypti and Aedes albopictus mosquitoes, took off and the four varieties of the virus settled in.
 Harris first went to Nicaragua in 1988 after graduating from Harvard. As an activist with a privileged education, she arrived with a head full of ideology and a determination to go to work. “I wanted to put my money where my mouth was,” she says with a laugh. She’s a bubbly woman with smiling hazel eyes, well-kempt curly brown hair, and colorful accent scarfs. Inside her hectic UC Berkeley office, strewn with paperwork stacks, mementos and notes, she talks fast, but with purpose. Harris first went to Nicaragua in 1988 after graduating from Harvard. As an activist with a privileged education, she arrived with a head full of ideology and a determination to go to work. “I wanted to put my money where my mouth was,” she says with a laugh. She’s a bubbly woman with smiling hazel eyes, well-kempt curly brown hair, and colorful accent scarfs. Inside her hectic UC Berkeley office, strewn with paperwork stacks, mementos and notes, she talks fast, but with purpose.
When she first arrived in Nicaragua, the revolution and Contra war were still raging. Soldiers were returning to Managua with jungle diseases unfamiliar to urban clinics, such as the skin parasite leishmaniasis. Overwhelmed and clutching photocopied pages from an undergraduate molecular biology textbook, Harris sweated in the Ministry of Health’s labs.
Her passion to help Nicaragua became incurable after that inaugural trip. As she worked through graduate school at Berkeley and into a faculty position, she found a way to go back each year and help build resources and train scientists. Her first triumph was devising lab techniques to work without electricity—techniques to diagnose leishmaniasis. When she asked what they needed next, health workers rattled off a list topped with dengue. By 1995, it was her primary focus.
 “I was completely winging it,” Harris says. When she returned home in 1997, broke, she learned she had won a “genius grant” from the MacArthur Foundation, worth $210,000 over five years. “I literally didn’t have money to pay the rent, so that was good,” she says. “I was completely winging it,” Harris says. When she returned home in 1997, broke, she learned she had won a “genius grant” from the MacArthur Foundation, worth $210,000 over five years. “I literally didn’t have money to pay the rent, so that was good,” she says.
Moreover, her Nicaraguan colleagues embraced her efforts. She picked up Spanish quickly, drawing on the French she learned growing up. And she readily fit into the colorful culture—particularly the strong tradition of dancing. “Eva loves dancing. She’s the dancing scientist,” says Josefina Coloma, director of the Sustainable Sciences Institute, a non-profit set up by Harris to help communities in developing countries build resources. “It’s how she relaxes,” Coloma says.
The dengue war within
Dengue’s common name may come from the remnants of “evil-spirit sickness” in Swahili, ka-dinga pepo. But in Nicaragua, dengue fever goes by Quebranta huesos—“break-bone fever.” After a mosquito drops off the virus, it usurps cells in the bloodstream and takes laps through the circulatory system. The body counters with a firestorm of blazing inflammation and activated immune cells, leading to the namesake fever, and sharp pains in muscles and joints. Eighteenth century recounting of dengue cases noted wretched gaits of victims as they swung swollen limbs.
For some who get dengue that will be all; for others, it’s just the beginning. The incendiary inflammation can singe the lining of capillaries—the thinnest blood vessels—creating rifts between cells for blood plasma to seep out. With systemic internal bleeding, blood pressure drops, shock sets in, and death can come quickly from organ failure.
Since there’s no dengue-specific treatment, doctors combat severe disease by compensating for lost fluids, like constantly pumping up a tire with a puncture. They have to keep the patient’s blood pressure high enough to circulate blood. But too much could “pop” the system, causing life-threatening edemas that saturate tissues such as the lungs or brain. In Nicaragua, babies and young children get severe disease the most—and it’s on the rise.
To find out why, Harris and her team studied the body’s first-responder immune cells. They focused on B cells, the watchmen in the bloodstream that trigger the immune system’s alarms when an invader is detected. “They are the ones that actually make the antibody—the small molecules that will help kill the virus,” says Simona Zompi, an immunologist working with Harris. Antibodies make the difference between protection and an overzealous response.
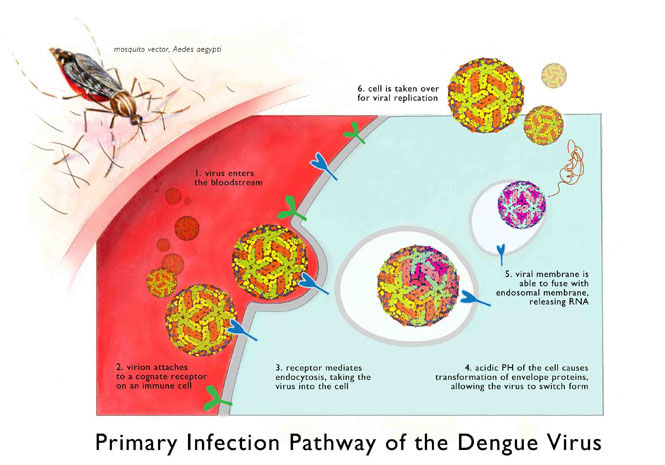
Illustration: Nicole Wong
(Click on image to see larger version)
|
|
|
Dengue viruses invade cells by latching onto specific receptors that coat the cell’s outside surface. The cells absorb the clinging viruses and enclose them in a sac where they’ll digest them with enzymes, like a piece of food in a stomach. But dengue virus can set itself free using proteins that cut open the sac. After its Houdini-like escape, the virus commandeers the cell to replicate itself.
The antibodies, deployed by B cell watchmen, can tackle the virus before it gets a chance to invade. Antibodies blanket the virus and signal cells to digest the invader while preventing sneaky escapes. But this security system depends on the type of antibodies called into action. “There are some good antibodies and some bad antibodies,” says Zompi. “The bad antibodies can make it worse.”
Bad antibodies that only partially recognize the virus cause a berserk immune response. The few bad antibodies clinging to the virus will signal the immune cells to destroy, but without complete antibody coverage, the virus can escape. Potentially all immune cells responding to the invader will become dengue’s patsies. The bad antibodies inadvertently help the virus infect more cells, and the inflammation response will crank to an inferno level.
The soul of the fight
On a boiling February day in 2012, seven hundred Managuans swarmed for an annual celebration of community efforts to kill dengue. Donning hand-sewn mosquito costumes and shirts showing a mosquito lifecycle being snipped, each neighborhood’s brigadistas paraded into a city pavilion. They carried banners saying “We can do it!,” played music and danced. “Every barrio comes in—all 30 of them,” Harris says. “There’s this monstrous amount of excitement.”
On a concrete slab under a pavilion, the teams have a full day of mosquito life-cycle skits, Reggaeton dancing, and awards for the best team leaders. Harris sat in the front row with colleagues and donors—reserved Swiss bankers—watching scantily clad Managuan women gyrate to the hip-hop music. They eventually pulled her on stage, where she held her own with the dance moves. Each year, there’s also a hip-hop song specifically written for Harris. One, by Israel Rodriguez, went:
“We have to cut the life cycle of the 'little nails'
If you’re not careful they’ll give you dengue.”
To community organizer Harold Suazo, this is the only way dengue control will succeed. “It doesn’t work to come from the outside, but from within—the soul of the barrio.”
 The communities have embraced Harris’s research as well. Four thousand children enrolled in her studies. Many are in a “cohort” study, in which researchers check the children each year for new infections and the kinds of antibodies scanning their blood. Children start the program as early as six months old; they remain subjects until they turn 14 or 15. The study is now in its eighth year, making it the longest such project in the world. The communities have embraced Harris’s research as well. Four thousand children enrolled in her studies. Many are in a “cohort” study, in which researchers check the children each year for new infections and the kinds of antibodies scanning their blood. Children start the program as early as six months old; they remain subjects until they turn 14 or 15. The study is now in its eighth year, making it the longest such project in the world.
“There’s really interesting data coming from Harris’s group,” says infectious disease specialist Thomas Jaenisch of University Hospital Heidelberg in Germany, who runs global dengue clinical trials. Jaenisch isn't aware of other groups that could answer questions about cycles of the disease in children.
Zompi and Harris also compare who’s been infected with who has gotten symptoms. “If you want to develop a vaccine, that’s what you want a vaccine to look like—a person who can get infected but doesn’t develop symptoms,” Zompi says. In a new study, when a child comes to the hospital with dengue, health workers fan out in that child’s neighborhood to fetch asymptomatic patients. “We will go and knock on doors,” Zompi says. “We say, 'Hello, we would like to take your blood. You’re doing fine, but….'”
Based on statistics from previous years, about 3 percent of symptom-free neighbors will also be infected. But neighbors must give blood three times; the last requires a voluntary trip to the clinic 15 to 28 days after the first. When the researchers did a trial run in 2006, 89 percent of the neighbors showed up. “They see [dengue] like a big burden,” says Zompi.
By the time many kids reach nine or ten, they’ve been infected three or four times. Since 2004, the number of children in the cohort study with severe disease mysteriously jumped from 20 percent each year to 60 percent.
The antibody lottery
To reveal what’s driving the disease spikes, Zompi figured out how to induce a berserk immune response in mice. She infected them with one type of dengue virus, then injected an antibody designed for another type, mimicking the cycles of dengue in people. (See sidebar: Weeding Out the Path of Disease.)
In other countries where dengue has been endemic for years, the four types of dengue are balanced, infecting almost equally. But in Nicaragua and other recently infested Latin American countries, Harris’s cohort study found that the viruses take turns infecting. Right now, dengue type 3 reigns in Nicaragua. Before, it was type 2. A few years before that it was type 1.
 The pattern turned Zompi’s attention to a special antibody-making cell: memory B cells, which produce antibodies based on past infections. In infections with a cousin of dengue, the West Nile virus, researchers found that memory B cells make different antibodies than those scanning the bloodstream. In Managuan children, Zompi says, memory B cells might have made good antibodies against type 1 dengue. But when type 2 dengue took over infections, the epidemic of severe disease started and the cells instead made bad antibodies. The result: a berserk immune response and severe disease. The pattern turned Zompi’s attention to a special antibody-making cell: memory B cells, which produce antibodies based on past infections. In infections with a cousin of dengue, the West Nile virus, researchers found that memory B cells make different antibodies than those scanning the bloodstream. In Managuan children, Zompi says, memory B cells might have made good antibodies against type 1 dengue. But when type 2 dengue took over infections, the epidemic of severe disease started and the cells instead made bad antibodies. The result: a berserk immune response and severe disease.
Though it solves the mystery of severe disease spikes, the finding complicates the hunt for a vaccine. If a vaccine activates antibodies from memory B cells, researchers would need to know if those antibodies will prevent disease or make it worse, Zompi says. She and Harris are collaborating with the National Institutes of Health to look at a promising vaccine in early trials in the U.S. and Colombia. But vaccine volunteers in the U.S., where there’s no dengue infection, don’t have memory B cells waiting in the wings. In Colombia, which is behind Nicaragua in the dengue epidemic, there’s a mix of dengue exposure.
For Nicaraguans who have already been infected, most many times, the vaccine might be too much of a gamble. It could cause memory B cells to produce bad antibodies years down the road, says Zompi—reigniting the sparks in severe disease we’re seeing now.
The dengue road map
As Latin American countries struggle with their new invaders, dengue cases are popping up on the fringes of its range. The U.S., France, and Croatia have all seen clusters of infection recently. “We don’t have a lot of data in Africa, but I’m sure it’s there,” Zompi says. Even the data for countries infested with dengue are unreliable, Harris points out.
“Dengue is hard to diagnose,” says University of Oxford epidemiologist Simon Hay. “People don’t have the blood tests to do it, and instead they lump it into other fever-inducing diseases.” Hay is leading an Oxford team to answer a 2008 call by the European Union to create a risk map of dengue spread. The team, which holds expertise in mapping malaria outbreaks, will assemble maps of where dengue might be in 2020, 2025, and 2080.
“We don’t know what the global burden of dengue is today, which is pretty much a travesty in its own right,” Hay says. The team is gathering case studies and medical reports to piece together which countries have dengue and, of those, how severely each is infected. The first goal, he says, is to come up with a ranked list of countries facing the deadly virus. Next, they’ll use today’s map to understand dengue-friendly environments and predict where they’ll be in the future. We need to know how warming climates and city sprawl will change the map down the road, says Jane Messina, the head medical geographer on the project.
Regardless of the changes, the road will be rough. “These maps are usually badly received,” Hays admits. Global politics and financing are just some of the reasons that politicians and institutions balk at risk maps. But Hay hopes the burden numbers will raise the heat on global funds to give to countries facing dengue a chance to fight. Given the resources, the people on the ground will know how best to stop the scourge, Hay says.
Back in Managua, community organizer Harold Suazo agrees. “The foundation of dengue control is social capital, it’s social networking, it’s leadership, it’s people,” Suazo says. When people do things because they love it, they put everything into it. That’s the heart of dengue control.
Sidebar: Weeding Out the Path of Disease
Nestled in the rolling hills north of San Francisco, the UC Hopland Research and Extension Center is home to flocks of irksome turkeys, scurrying lizards sporting tag numbers, and a blanket of golden pasture speckled with plots: plots of disease. Researchers are using the snippets of grassland and a virus, called Barley Yellow Dwarf Virus, to decipher the formula for epidemics.
Before exotic diseases explode into epidemics, they often hop from animals and leapfrog through communities. Researchers from the University of North Carolina think that plagues—like flu, Lyme disease, and dengue—spread by having just the right mix of potential victims in a community. Each victim along the way—chickens, mice, monkeys, humans—can be assigned a disease transmission score that describes how easily they’ll get, carry, and ultimately transmit a disease, says lead researcher Miranda Welsh, a graduate student at UNC. The community’s tallied score could determine a plague’s staying power.
|
Beth Mole describes research to trace how disease epidemics arise, using plots of virus-infected grasses in northern California. (Click on image to see video.) |
|
|
To test their hypothesis without unleashing diseases on animals, Welsh and her colleagues turned to the model grass system to map the trail of aphid-spread BYDV. Just as mosquito bites might lead to dengue or malaria, sap-sucking aphids transmit BYDV to grasses, causing yellow wimpy blades. In two-and-a-half-foot square plots, they created mixed “communities” using eight species of grasses—each with a different transmission score. “The experiment just looks like a big lawn,” Welsh says, laughing.
The eight species exist on a spectrum from low transmission score—“slow return”—to high—“quick return.” The quick-return grasses grow fast, die quickly, and burn through energy with fired-up photosynthesis. These species, mostly annual grasses, typically host the most disease. They’re like mice, which are reservoirs for plagues such as Lyme disease. Slow-return species, such as perennial grasses, typically tolerate less disease in their populations over time, like primates.
A formula for an epidemic, Welsh suggests, might be many quick-return species alongside slow returners. The prediction seems to play out in natural communities, she points out. In Mast Oak years—when oak trees produce an abundance of acorns for mice to nosh—the subsequent bursts in rodent populations correlate with Lyme disease outbreaks.
The research team will test their formula over the next few years in the grasslands. “In the end, we want to know, ‘What’s our chance of getting malaria? What’s our chance of getting dengue?’” Welsh says. Until we understand a disease’s path to an epidemic, we’ll be lost in the weeds.
Story ©2012 by Beth Marie Mole. For reproduction requests, contact the Science Communication Program office.
Top
Biographies
 Beth Marie Mole Beth Marie Mole
B.S. (biology and ethnomusicology) The College of William and Mary
Ph.D. (microbiology) The University of North Carolina at Chapel Hill
Internships: The Chronicle of Higher Education (Washington, D.C.), The Scientist, Nature
When I was an undergraduate, it was how bacteria in our gut teach us about our ancestors. As an environmental camp teacher, it was field trips to wastewater treatment plants to watch microbes transform goopy sewage into clean water. In graduate school, it was a bacterium that quick-changes from a halcyon detritus grazer to a ruthless plant assassin. And after my doctorate, it was bacteria that defeat antibiotics.
I loved it—all the fascinating its of the natural world. For years they were microbes and their adaptations, but now they’re global public-health issues and compelling interdisciplinary challenges—how geographers improve vaccines, what grasses reveal about the spread of malaria. Their endless possibilities led me from the bench to the writing world. I love sharing them as much as exploring them—the next its.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
 Maayan Harel Maayan Harel
B.S. (geophysics/atmospheric science and political science) Tel Aviv University, Israel
Internship: Smithsonian Institution National Museum of Natural History (Washington, D.C.)
I always assumed I would pursue art as a career, but along the way other interests took hold as I gained more life experience. I was in the military, backpacked across Asia, and became fascinated with earth sciences. Working as a researcher in climate modeling led me to the realization that science communication is of vital importance, and I am excited about using art to express scientific ideas and research. This career allows me to maintain the sense of wonder I felt as a child exploring the natural world. Please check out my website.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
 Nicole M. Wong Nicole M. Wong
B.S. (conservation and resource studies) University of California, Berkeley
Internship: American Museum of Natural History (New York)
I have a fondness for all animals, from the charismatic to the positively disgusting. I hope my illustrations elevate their stories and place them in a new light. For more examples of my work, please visit my website.
Top |