|
Twinkle, Twinkle Little Cell
A technique borrowed from astronomy is sharpening microscope images. What will biologists see? Adam Mann clears things up. Illustrated by Arianna Bruno and Annette Filice.
 Illustration: Arianna Bruno Illustration: Arianna Bruno
The Earth’s atmosphere is an ocean of air. It churns, gushes, and incessantly flows above us. Anything passing through this pandemonium is buffeted about, including starlight. A star’s twinkle is merely a larger version of a candle flickering in the wind. While this may make for good nursery rhymes, it’s bad for astronomy. Subtle features in telescope images are washed out, and distant objects appear smudgy.
The briny fluid within cells also flows in minuscule swells and surges. This ever-changing activity blurs light coming from the deepest regions. Biologists peering into a cell’s center see unfocused specks. Just beyond the current reach of microscopes, a mere half-millimeter away, lies a mysterious world.
During the last 20 years, astronomers learned to subdue the atmosphere’s billows. Using a technology called adaptive optics, they created an artificially steady night sky and cleared up telescope images. With a guiding beacon of light, they can turn misshapen blobs into galaxies merging, stars orbiting one another, or gas swirling around massive black holes.
Now, biologists think they can take the same leap. In a small lab at UC Santa Cruz, researchers are manipulating tiny glowing spheres to illuminate the inner workings of a cell. Using expertise from genetics, physics, and engineering, the team hopes to give microscopes greater clarity. They aim to watch chromosomes dividing, neural connections growing, and stem cells multiplying in real time.
But the future of adaptive optics in biology remains hazy. No one yet knows what it will take to make the technology mature—and what will appear when it’s ready.
“Sometimes you just have to build a great new tool and see what comes out of it,” says astronomer Claire Max, director of the Center for Adaptive Optics at UCSC.
As one of adaptive optics’ first advocates in astronomy, she should know. During the 1970s, Max was a consulting scientist on classified espionage projects for the U.S. military. At the time, the Soviets were launching one satellite per day, making the Americans nervous, she says. Max and her colleagues focused adaptive optics telescopes on these orbital fliers to garner hints about their function. For instance, she explains, a satellite without solar panels was probably nuclear powered. The military used these details to keep tabs on Soviet activity in space.
Max and atmospheric scientist Robert Fugate of the U.S. Air Force knew that adaptive optics had potential for civilian use. By circumventing the atmosphere’s turbulence, they could equip astronomical telescopes to see distant stars with unparalleled detail. But they needed a new source of light to point the way.
Guiding lights
A toy ball resting at the bottom of a pool looks blurry because of the water’s movements. Adaptive optics lets researchers ask: What would the ball look like if there were no water? By contrasting the ball’s wavering image to how it should appear without distortion, they trace how much the light is bent and calculate the water’s ebbs and flows.
In an adaptive optics system, a computer linked to a telescope or microscope crunches the distortion data. The computer then tells tiny pistons to move a flexible mirror, which undulates thousands of times per second in response to the changing environment. Each adjustment shifts the surface by just the right amount to cancel out the warping of light. With the distortions corrected, a scientist can point their scope to view any object with clarity.
But until the 1980s, astronomers using adaptive optics needed extremely bright stars for the technique to work. Bright stars produce enough light to plow through the chaotic atmosphere without severe distortion. It is like a large ball at the bottom of a pool, which appears fairly solid, compared to a smaller ball, which the water’s movements will completely warp. Unfortunately, only one percent of the night sky contains stars bright enough for adaptive optics computers to analyze.
To free astronomers from this constraint, Max and Fugate decided to make their own star. They beamed a yellow laser at layer of sodium atoms high in the Earth’s atmosphere. The laser excited the atoms, causing them to radiate and produce a sharp dot of light. This artificial “guide star” could be swung around to probe any sector of the night sky.
Working at Lick Observatory near San Jose, California, Max and Fugate tested and developed their guide-star-oriented adaptive optics system. In the early 1990s, when the military work was declassified, other astronomers gained access to the technique and helped to mature the technology. These days, a half-dozen or so telescopes use laser-guided adaptive optics—including one of the world’s most powerful, the Keck Observatory in Hawaii.
With adaptive optics, astronomers have enhanced their view of the cosmos. Fuzzy blotches become galaxies in the early universe crashing together and assembling into larger units. Indistinct smudges turn out to be orbiting asteroid pairs, whose size and composition gives insight into how our solar system formed. And what was once a haze of light transforms into stars rotating at the center of the Milky Way, producing information about what created the black hole that lies there.
The unseen world
Telescopes and microscopes uncover what was previously invisible to the human eye. Reworking adaptive optics from one to the other seems obvious enough. Yet there are key differences. For instance, resolving the surface of a cell is trivial. “But if you go down 30 microns, it looks like crap,” says developmental biologist Bill Sullivan, who leads one half of the adaptive optics microscopy team at UCSC.
Sullivan has spent his career investigating fruit fly embryos, turning genes on and off to determine their function. He likens a developing cell to a car factory and genes to workers on the assembly line. Tying the hands of one worker and waiting to see what the resulting cars lack—a missing door or roof or steering wheel—tells him which worker builds which part.
Sullivan is trying to understand how a widespread parasite, the bacteria Wolbachia, works on its host. Research suggests that Wolbachia is one of the world’s most common parasitic microbes, infecting between one-quarter and three-quarters of all insect species. The parasite renders a species sterile, either by selectively killing males or turning them into females. Unraveling how Wolbachia controls a species’ reproduction could lead to protection against nematode diseases in humans and may help suppress mosquito populations in malaria-filled areas.
Sullivan’s experiments explore how Wolbachia influences a fruit fly embryo’s nucleus—the round compartment that holds a cell’s DNA—during division. Just after fertilization, this compartment splits into two nuclei, then four, then eight, and so on. After the seventh division, the nuclei migrate to the cell’s surface and float like lily pads on a lake.
But before this, the cell’s fluids conceal any activity. “We want to see what happens when the nuclei bang into each other,” says Sullivan. “But the cell is all full of this yolky cytoplasm.”
He could kill the embryo at stages prior to the seventh generation and slice it open to inspect the innards. But that would be like trying to determine the dynamics of an ecosystem from frozen photographic moments. The real-time activity would remain a mystery. So Sullivan wanted a method to transform the cytoplasmic haze into an unclouded fluid.
For years, Sullivan passed the Center for Adaptive Optics on the way to his lab. One day it struck him that the center’s work might be useful in his research. He talked to astronomers, asking for help with his problem. Engineer Joel Kubby heeded his call.
“I understand things by seeing them,” Kubby says. “Working on different forms of microscopy to see better has been a theme of my career.”
In the early 1980s, he received his doctorate from Cornell University in applied physics and then found a job at Bell Laboratories. There, he imaged germanium surfaces used to make the first Bell Labs transistors. The work employed the newly invented scanning tunneling microscope, a machine that allowed scientists to see objects at the nanoscale, the size at which atoms and molecules operate.
After spending years in industry, Kubby wanted to get back into academic research. He got a position at the Center for Adaptive Optics to develop new microelectrical mechanical machines (MEMS)—nanoscale devices that promise a superior alternative to current instruments.
A deformable MEMS mirror is less than a half-inch wide, while previous flexible mirrors were generally between four and six inches. MEMS instruments are mass-produced, making them ten times cheaper than current $250,000 mirrors. As well, MEMS systems are accurate. Rather than clunky pistons, MEMS mirrors use tiny, precise devices driven by electrical currents that move the mirror’s surface by an amount smaller than a speck of dust.
Candles in the dark
Though other researchers are working on adaptive optics in biology (see sidebar: Seeing Eyes), the Santa Cruz effort is unique. In order to rid a fruit fly embryo of visual aberrations, Kubby restyled the guide star trick from telescope operators.
“Rather than reinventing the wheel,” he says, “it’s better to take the wheel that’s there and adapt it for what we’re trying to do.”
Instead of artificial stars, Kubby’s team injects hundreds of microscopic fluorescent spheres into a cell. These glowing dots create a band of tiny “flashlights,” illuminating the surroundings for the adaptive optics system. By locating one, the team can take a photo at any point within the cell’s depths. The work has paid off; the researchers have achieved a ten-fold increase in resolution, greater than any other team developing adaptive optics microscopy.
Prepping the samples this way is complicated. The specimen first needs to be carefully dried out in order to accept a bead-filled fluid, killing the cell and ruining the chance to observe changes in a living organism.
“We’d like to eliminate the need for the bead,” says Kubby.
To accomplish this, Sullivan inserted a gene originally found in jellyfish into fruit fly embryos. It codes for a green fluorescent protein (GFP), which emits a small spark of bioluminescent light. The scientists know that these GFPs will provide enough light to clear up images without destroying the sample, but they still must breed embryos for this purpose.
A competing team at the Howard Hughes Medical Institute’s Janelia Farm Research Campus in Virginia has taken a different approach. Instead of physical beads, engineer Eric Betzig and physicist-turned-neuroscientist Na Ji measure distortion by breaking a ray of light into many separate beams and shining them on their sample. They compare images taken with each distinct beam to that from the total. Any aberrations will stick out clearly.
Betzig and Ji's work investigates the outermost brain region, an area known as the cortex that is thought to perform calculations associated with memory and learning. The team looks at the cortexes of mice, which are organized into six layers that compute via a complex and poorly understood network. Currently, Ji and her colleagues can only see neurons turning on and off in the topmost layers. "In a way, it's like trying to understand how a CPU works by only measuring 20 percent of the electric circuit," she says, adding that imaging deeper with adaptive optics will hopefully help them understand how the circuit works.
Ji hopes to use adaptive optics to watch individual neural connections grow and change in the mouse's brain during learning. This may ultimately help researchers pinpoint how the brain calculates information and lead to a better understanding of the cortex's workings, she says.
But Kubby says the HHMI research team will always be too slow to create real-time images and video. He feels his technique will yield superior results since it is faster—and, he notes, it already works for astronomers.
Kubby and Sullivan’s big advantage is calling on the expertise of the Center for Adaptive Optics. “We’re miles ahead of a medical school because they don’t have an astronomy department,” says Sullivan.
For now, the Santa Cruz team has shown that GFPs are bright enough for adaptive optics work. The final step is to arrange all the pieces together—embryo, GFP, MEMS mirror, and adaptive optics—and produce images of dividing nuclei within the developing fruit fly.
After that, the researchers hope to extend their methods to other areas of biology. UCSC biologist Lindsay Hinck, who studies breast cancer, eagerly awaits the team’s first results. Mammary glands, she explains, need to completely reconfigure themselves to produce milk, so they contain a high concentration of stem cells. Scientists think stem cells reorganize breasts by dividing asymmetrically, resulting in two different cells—a mammary cell and another stem cell. But tissue and other cells protectively surround stem cells in the body, making their behavior somewhat mysterious.
“No one has ever been able to see an asymmetric cell division,” says Hinck. “The resolution is too poor.”
She hopes to do real-time imaging, observing these rare splits rather than the nebulous cellular collections. Such data should yield insights into stem-cell behavior and what causes them to go awry, developing into tumors.
Seeing: the future
For all the potential applications of adaptive optics microscopy, this new tool has yet to take its first scientific images. Biologists have a “show me” attitude, says Joel Kubby, with most waiting until the instrument produces data-rich pictures and information. “When you figure out new science,” he says, “that’s the real home run.”
If the team brings the technique to fruition, Bill Sullivan hopes that it will become a staple for biologists. Twenty years ago, he says, everything in biology involved fixed imaging. Now live imaging has become the norm. The next frontier, he says, could be live imaging deep inside cells.
With adaptive optics, biologists will cut through flickering fluids and probe their samples for once-hidden phenomena. The technology, which has already expanded our perspective on the cosmos, is set to open up a new world of exploration in biology.
Seeing Eyes
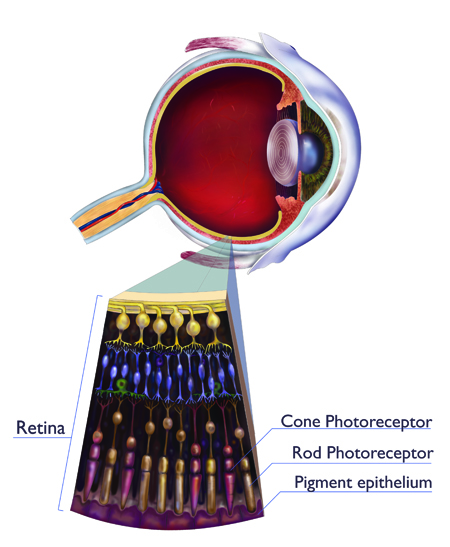 |
Illustration: Annette Filice  |
Austin Roorda has seen many eyes. As a vision science researcher at the University of California, Berkeley his career has focused on how eye diseases progress. A decade ago, he was one of the first scientists to turn the power of adaptive optics on the human eye. Now that the technology is mature, Roorda says it allows vision scientists to identify maladies and develop drug treatments; someday, it may help combat the early stages of blindness.
Ten years ago, psychologist David Williams developed the first adaptive optics retinal imaging techniques at the University of Rochester. Roorda, a student under Williams, used it to look for microscopic blemishes on the retina. The technology impressed him.
“For the very first time, we could measure properties of individual cells in a living eye,” he says.
Adaptive optics retinal cameras use the same “guide star” technique as astronomers. Researchers shine a weak infrared laser into a person’s eye. Light bouncing off the retina reveals how the lens fluctuates over time. A computer and flexible mirror then erase the distortion.
Roorda’s goal with adaptive optics is to understand the changes that occur in the eye from diseases, particularly rare ones. Using donated eyeballs, he is studying an unusual disorder called macular telangiectasia, in which blood vessels penetrate the retina and cause blindness. Unfortunately, only one patient with the malady has ever donated his eye to science—“one precious retina used to understand this disease,” says Roorda.
But with adaptive optics, ophthalmologists can study the disease as it progresses in living people. Roorda is exploring what comes first: the photoreceptors dying, or the blood vessels penetrating. “We now have the tools to answer those fundamental questions,” he says.
Adaptive optics is helping ophthalmologists in other ways. Most eye diseases progress slowly, and the techniques used to measure their development are crude. Companies typically avoid the costly time and money investments required to develop helpful drugs. Adaptive optics gives them a new device to make detailed images of a retina. By imaging that same retina over the course of a drug trial, they can see how effective a treatment is.
If adaptive optics becomes cheap and widespread in optometrist’s offices, doctors will also be able to see the early warning signs of age-related blindness. One day, Roorda speculates, an optometrist will prescribe a drug to patients who begin exhibiting signs of macular degeneration. In this vision of the future, age-related blindness will become manageable.
Story © 2010 by Adam Mann. For reproduction requests, contact the Science Communication Program office.
Top
Biographies
 Adam Mann Adam Mann
B.A. (astrophysics) University of California, Berkeley
Internship: Nature (Washington, D.C.)
When I was an undergraduate studying astrophysics, I had trouble admitting to my creative side. After all, scientists are logical, analytical people who enjoy the thrill of pure research. I was not one of those scientists. In the lab I was a passive participant, my mind constantly wandered, and I soon realized that I needed something more. . . creative.
There’s that word again. The one I wasn’t supposed to admit to. But I knew it was inside of me and, after spending several years traveling, teaching, and experiencing the world outside of science, I was ready to own it. With science writing I can be an actor and not just an observer. I can engage in a fascinating world of information and simultaneously share what I learn with others.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
 Arianna Bruno Arianna Bruno
B.S. (biology) East Tennessee State University
M.A. (biology) Smith College
My goal as a scientific illustrator is to inspire appreciation, especially appreciation of small creatures and details that typically go unnoticed. Like many of my colleagues, I have a fondness for the intricacies of nature, and I love how illustration allows me to share what I see and what I learn. The Science Illustration Program has been a great experience for me. It has given me the instruction, focus, and guidance to develop as a professional artist, and I am excited to begin my new career. In the fall, I will be exploring scientific sculpture, another aspect of science illustration, and the intricacies of the human face as an intern at a facial prosthetics clinic at The Johns Hopkins University School of Medicine.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
 Annette Filice Annette Filice
B.A. (biology) California Polytechnic State University, San Luis Obispo
The first canvas I ever worked on was the stark white wall of my childhood kitchen, the choice medium being my mother’s bright red designer lipstick. Needless to say, I have always loved to draw and paint (with anything I could get my hands on). Growing up in San Jose, I witnessed the effects of urban sprawl on both environment and community. Consequently, my education gravitated toward conservation.
As a biology undergraduate at Cal Poly, I worked on a number of botanical illustrations for a lab manual, and realized that art science hold great potential to work cohesively. As a science illustrator, I hope to inspire others to reconnect with plants and animals in our environment that sustain human life. Attending the Science Illustration Program at CSU Monterey Bay has given me excellent training in illustration techniques, and a strong appreciation for sketching in the field. Whether in your garden or high in the canopy of a redwood forest, Earth’s natural beauty provides infinite creative inspiration.
Top |

