Mercury Rising
Restoring the wetlands of San Francisco Bay means better flood control, more wildlife habitat—and, some worry, more toxic mercury. Sandra Chung reports. Illustrated by Sarah McNaboe.
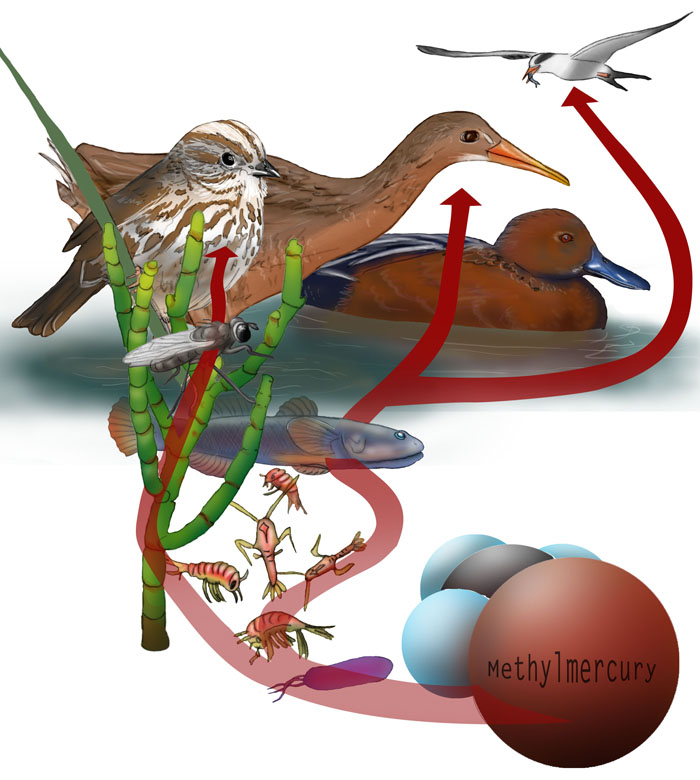
Illustration: Sarah McNaboe
ALVISO, CA—Nine miles from the heart of Silicon Valley, a rumpled young brown-and-white heron emerges from the marsh grass and blinks at the cinnamon-colored ducks zipping by in Mallard Slough. It’s low tide, and all the muddy rivers in the area are emptying into the southern part of San Francisco Bay.
Just a few years ago, a bleak expanse of grey salt crystals dominated this landscape. Today, the second-largest wetlands restoration project in the country is slowly returning these former salt production ponds to the lush marshes they once were. The restored wetlands will protect and benefit people and wildlife alike.
But one toxic legacy of human presence lingers in the water, sediment, and air here. If the restoration managers and scientists can’t keep it in check, it could take a tragic toll on the very wildlife they aim to help.
Long before it became part of Silicon Valley, San Jose was a mercury town. Throughout the latter half of the 19th century, Mexican and Chinese miners hammered and blasted bright red chunks of cinnabar out of the New Almaden mine, southeast of the city. The silvery liquid that the miners burned off the red rocks was nearly pure elemental mercury, coveted by gold miners who used it to pull the precious yellow metal out of the ore they hacked out of the Sierra Nevada.
The Guadalupe River still carries New Almaden mercury fourteen miles to the southern part of San Francisco Bay via Alviso Slough. During the mine’s heyday, the river laid down a layer of heavily contaminated sediment several feet thick. Reconnecting the salt ponds to the Bay could churn up the mercury buried in these sediments and activate a 160-year-old stockpile of toxic metal.
Most of the mercury that poisons birds, humans and large mammals comes from their diets. Under the right conditions, common bacteria convert inorganic mercury into an organic form, methylmercury, which can enter the food web. Methlymercury concentrates in living tissue as it travels up each level of the food web.
The South Bay is a methylmercury hotspot. The average concentration of methylmercury in the water here is four times higher than the Bay average. And the salt ponds are part of the problem.
“They're almost perfect methylmercury generating engines in their current form,” says Collin Eagles-Smith, an ecologist for the U.S. Geological Survey (USGS). “They also happen to host some of the largest breeding colonies of waterbirds in the South Bay."
Turning the ponds back into marshes will bring even more wildlife, fish and plants to the South Bay. It could also expose them all to higher levels of toxic methylmercury. “When you make marshlands, you make just the perfect conditions for the microbes that convert mercury into methlymercury,” says Russ Flegal, an environmental toxicologist at the University of California, Santa Cruz. “By doing this thing to really improve the health of the Bay, we may really be making the methylmercury concentrations in the Bay much higher.”
Scientists don’t know what long-term effects the wetland restoration will have on mercury in Bay waters and wildlife. The chemical and physical processes that transform mercury into methylmercury are too complex for the restoration team to predict what will happen when they reconnect the salt ponds to the Bay. The project managers are well aware that they could set off a mercury time bomb if they proceed without care. They cannot simply open the floodgates and hope for the best.
Fending off a perfect storm
In the 1850s, 190,000 acres of wetlands lined San Francisco Bay. Developers diked and drained more than 96 percent of them to create waterfront property and evaporation ponds for salt production. “We've pretty much built on our wetlands,” says Don Yee, an environmental scientist at the San Francisco Estuary Institute, which acts as a science consultant for the marsh restoration project. “There are now million-dollar homes sitting pretty close to sea level."
Much of that real estate could be underwater within a few decades. Climate scientists predict a 3-foot to 5-foot rise in sea level in the Bay Area by the end of the 21st century, as well as even larger changes in the tides and in the frequency of severe weather.
In addition, some areas are as much as six feet lower than they were before the region’s farming heyday in the mid-20th century. Free-flowing rivers once helped maintain the land elevation by continually depositing sediment. But damming rivers upstream to provide irrigation water for agriculture left the downstream Bay dirt-poor, so to speak. And pumping water from the ground to irrigate orchards left underground spaces into which the land on top slowly collapsed. The combination of rising sea level and sinking land elevation leaves the Bay Area susceptible to flooding.
"We'll probably take the Dutch approach and build higher and higher walls,” Yee says. The Bay Area would then be vulnerable to a catastrophic levee failure like the one New Orleans experienced during Hurricane Katrina in 2005. “Except an earthquake will do it here. We don't need a hurricane,” Yee adds.
Restoring some of the coastal wetlands could help defend against rising sea levels and extreme weather. Dense marsh vegetation puts the brakes on storm and flood waters and acts like a giant sponge that soaks up excess water and slowly releases it. The combined braking and slow-release actions spread out the energy of rushing waters over space and time, lessening their destructive impact.
Wetlands also help protect the Bay from pollution. Urban pollutants, pesticides and fertilizers from nearly half the land area of California end up in the Bay after traveling down the great Sacramento and San Joaquin Rivers. Coastal marshes help filter out some of the pollutants before they clog up and contaminate the Bay.
Finally, the new wetlands will bring more life to Silicon Valley. The salt ponds overlap with the country’s first urban wildlife refuge, the Don Edwards San Francisco Bay National Wildlife Refuge. The refuge’s lush array of plant, insect and animal life stands in stark contrast to the pale concrete expanses of nearby Silicon Valley. Thousands of migrating birds stop in the refuge to rest or breed. The restored wetlands will expand their nesting and feeding grounds and provide much-needed habitat for native species, including the endangered California clapper rail and salt marsh harvest mouse.
The San Francisco Bay Wetlands Restoration Project will restore 40,000 acres of wetlands. The U.S. Fish and Wildlife Service spent 15 years planning the ambitious project, which will proceed piecemeal over the next 50 years. Only the ongoing Florida Everglades restoration surpasses the Bay project in size. As with the Everglades project, scientists worry that methylmercury levels in water and animals might rise with the restoration of the marshes.
View South Bay Salt Pond Restoration in a larger map
Mercury occurs naturally in rock and soil, and burning fossil fuels releases naturally occurring mercury into the atmosphere. Atmospheric mercury provides most of the metal for methylmercury production in the Everglades and in most aquatic ecosystems that lack an obvious man-made mercury source.
But the San Francisco Bay project, unlike the Everglades, happens to sit on one of the biggest historical caches of mercury in the New World. Between 1844 and 1976, miners dug more than 40,000 tons of mercury out of the New Almaden mine. Mountain rivers and streams continue to wash mercury from mines in the coastal mountain and Sierra Nevada ranges into the Bay. The methylmercury concentrations in many Bay sport fish are so high that California Fish and Game cautions people not to eat more than one or two meals of those fish from the Bay each month.
The South Bay combines a generous supply of mercury from New Almaden and prime conditions for generating methylmercury. The type of bacteria that convert mercury into methylmercury are nearly ubiquitous, but many different factors need to align in a methylmercury hotspot. In the South Bay salt ponds and in tidal marshes, cycles of flooding and drying expose mercury in the sediment to chemical processes that convert it to a reactive form. The tides bring in a fresh supply of plant matter, food for the bacteria. In the process of chowing down on the plants, the bacteria inadvertently take up the reactive mercury and convert it to methylmercury to get rid of it.
Workers drained ponds to create evaporation beds for salt production, which cut the ponds off from the Bay. When the restoration project reconnects them to the Bay, it should ameliorate some of the conditions that make the ponds such effective mercury-generating engines. But it will also make some of those conditions worse.
Counting chicks
The ponds are a convenient pit stop for birds traveling the Pacific Flyway, a major migration route that stretches from Alaska to Argentina. But USGS ecologists have already found evidence of mercury poisoning in some migrating birds, including some species that nest in and near the salt ponds. Collin Eagles-Smith and his USGS colleague Josh Ackerman have spent the past five years monitoring the behavior and mercury levels birds near the Bay. They caught adult birds in nets to take blood samples, and drilled tiny holes in the shells of unhatched eggs to sample the egg whites. They also measured mercury levels in several key fish species that comprise a large portion of the birds’ diets.
Rapidly accumulating mercury from the birds’ South Bay fish diets appeared to kill some of their eggs and chicks, much as the pesticide DDT once took a toll on the breeding of the endangered bald eagle. One migrating bird species, the Forster’s tern, happened to breed in the South Bay during a period that corresponded to high mercury levels in their main food fish. Their blood mercury levels tripled in the six to eight weeks between when they arrived in the area and when they laid their eggs.
As for the eggs, their mercury levels correspond to their mothers’ blood mercury levels during the week they spend growing inside of her. The eggs that didn’t hatch or that hatched into short-lived chicks had significantly higher mercury levels than the eggs that became healthy chicks.
 Photo: Sandra Chung
Photo: Sandra Chung |
An egret forages in south San Francisco Bay's mudflats, site of a project to restore thousands of acres of marshland. |
|
Mercury could have killed the eggs outright. It may also have turned the adult birds into neglectful parents. Parent birds need to turn eggs over periodically to ensure even incubation and to help position the embryo properly for hatching. A normal embryo should be oriented with its head tucked under its wing in the large end of the egg, with its legs folded underneath. Just like a breech position often keeps human babies from being born naturally, an upside-down position cuts a bird embryo’s chances of hatching by as much as half. Many of the eggs that failed to hatch contained both high mercury levels and improperly positioned embryos, suggesting that corresponding high mercury levels in the parents might have damaged their nervous systems and impaired their ability to tend their eggs.
Despite the results of the bird studies, Eagles-Smith and many other restoration scientists believe that the potential benefits of the restoration still outweigh the potential risks from mercury. “If we can provide a large boost in habitat for these wildlife species, a small increase in mercury is not likely to have as large a negative effect as the positive effect an increase in habitat will have,” Eagles-Smith says.
But the methylmercury chain is so complex and variable that it’s impossible to know exactly how the restoration project will change it. Methylmercury production varies widely from month to month and place to place. It varies so much that scientists haven’t been able to tease out the effects of early restoration efforts from normal variability, Eagles-Smith says. But the evidence suggests that an upcoming major restoration step could profoundly impact methylmercury levels in the South Bay.
Waters rush in
In mid-January 2010, the project broke ground at the levee separating Ponds A5, A7 and A8 from Alviso Slough. Breaching the levee in fall 2010 will reconnect the ponds to the Bay, the first step in restoring them to their naturally vegetated state. Restoration scientists know that Pond A8 contains sky-high mercury concentrations in water and sediment, some of the highest in the restoration area.
Methylmercury formation requires a complex interaction between fluctuating temperatures, tides, salinity, organic matter and the availability of reactive mercury. Much of the old mine mercury lies deep in the sediment at the bottom of Alviso Slough, in a nonreactive form that doesn’t contribute to methylmercury production. But breaching the levee could bring much of that mercury back to the surface.
The whole area is lower than sea level. Opening it up to the Bay will bring the Bay waters rushing in. Computer models show that the resulting larger, stronger tides will scour the bottom and sides of the slough and dig up some of the buried mercury, says Mark Marvin-DiPisquale, a USGS microbial ecologist.
The freshly unearthed mercury may also be more reactive. Marvin-DiPasquale and his colleagues tried mixing buried sediments with water from the slough to see how much of the mercury in the unearthed sediment would become reactive. The amount of reactive mercury increased 40-fold to 60-fold when the sediments hit slough water.
A huge increase in reactive mercury doesn’t guarantee that methylmercury production will increase as well. Scientists expect the methylmercury in Pond A8 to go down once the levee breach keeps the pond more consistently wet, cutting off the wet-and-dry cycles that encourage methylmercury formation. But the potential for the surging tides to trigger a methylmercury spike in other ponds warrants caution.
The most efficient way to keep an eye on the mercury problem is to focus on the animals themselves, says Letitia Grenier, a conservation biologist at the San Francisco Estuary Institute. Different plants and animals take up methylmercury at widely varying rates depending on their environment and behavior. Predicting mercury levels in organisms based on the conditions in their environment requires too many complex calculations and costly measurements to be practical, Grenier says.
Grenier led a three-year project with the Santa Clara County Water District to develop methods to monitor mercury levels in some of the most contaminated ponds. Her group decided the best strategy would be to look directly at methylmercury concentrations in three carefully chosen "sentinel" species. Longjaw mudsuckers live and feed exclusively in deeper water, brine flies in the shallows, and song sparrows in the marsh plains. A sudden uptick in mercury levels in any of the species could only come from a local surge in methylmercury. Like a canary in a coal mine, a regularly monitored sentinel species would quickly alert scientists to harmful methylmercury conditions.
Because no one has ever attempted to restore a complex wetland ecosystem on top of a historical mercury hotbed, the restoration project poses a unique opportunity to gather fresh data and immediately apply it. The site has hosted several ecological studies by the USGS and local universities, and project scientists have contributed to the decision to reconnect some ponds to the Bay and to keep some ponds separate. The mix of different ponds balances the need for more wildlife habitat with the potential risks of the restoration, such as mercury, says Laura Valoppi, the lead restoration scientist.
The project managers cautiously restored Bay water to Ponds 19, 20 and 21, the so-called island ponds, in March 2006. Before-and-after pictures of Pond A21 show fresh mud piling up and green pickleweed bursting out of the landscape like sprouts on a Chia pet. The appearance of thousands of shorebirds at the restored ponds roused enthusiasm about the future of the project. Even so, the restoration can’t completely erase the ecological consequences of more than 150 years of human industry and urban development.
“It's never going to look exactly like it did before,” Valoppi says. “But we can restore a lot of function and use for wildlife.”
The project will proceed slowly, restoring just a few hundred or a thousand acres at a time. Over the next 50 years, this wet, green wildlife oasis will gradually change the face of the Bay. But cautious scientists will have to test the waters—and the sediment, and the animals—at each step.
Story © 2010 by Sandra Chung. For reproduction requests, contact the Science Communication Program office.
Top
Biographies
 Sandra M. Chung Sandra M. Chung
S.B. (brain and cognitive science, biology) Massachusetts Institute of Technology
M.S.P.H. (environmental sciences and engineering) University of North Carolina at Chapel Hill
Internship: Idaho National Laboratory
I grew up believing I would heal sick people with my knowledge of science. I was a quiet, serious premedical student, but I became an animated joker whenever I had a chance to explain something to classmates and strangers. When I landed gigs teaching high school and undergraduate science courses, my time in the classroom and with students quickly supplanted my time in the lab. Through teaching I shed all remaining traces of shyness and developed a happy addiction to the "aha," the precious look on a student’s face when he grasps another small piece of the fabric of the universe. My own "aha" moment happened when my mother called me for medical advice. Why didn't she call my brother, the doctor? "You explain it so I can understand," she said.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
 Sarah McNaboe Sarah McNaboe
B.S. (environmental geology) University of California, Santa Cruz
Internship: Smithsonian Environmental Research Center, Annapolis, Maryland
I grew up overseas in Saudi Arabia, Indonesia and Kuwait. I moved back to the U.S. when I started college, and it was then as I was enrolled in both chemistry and art that I discovered scientific illustration as a career path. I graduated from UC Santa Cruz and went to work for an environmental consulting company for three years before coming to California State University, Monterey Bay, to attain my certificate in science illustration. It is my desire as a scientific illustrator to make science accessible to everyone. Visit my website, www.illustrationbysarah.com, to see more of my work.
Top |

