
|
| Illustration: Samantha Peters |
Virus fragments with a sugary finish may lead to a new vaccine against AIDS, reports Alison F. Takemura. Illustrated by Samantha Peters and Lindsey Kernodle.
On the wall above his computer screen, Phil Berman has taped a drawing of a molecule. It's an unusual hybrid: part protein, part sugar. The protein portion appears as a smooth ribbon, resembling a butterfly flapping by. The sugars sprout from the ribbon like brambles on the butterfly's back. The molecule looks innocuous, but in life, it's a deadly fragment. This is a small piece of HIV, the virus that causes AIDS.
Despite decades of progress, the human immunodeficiency virus still infects about two million new victims each year worldwide. About 95 percent of them are in developing countries. Berman, a biomolecular engineer who worked in the biotech industry before coming to UC Santa Cruz, thinks the fragment he keeps within glancing distance, and others like it, could be key to stopping HIV from spreading further.
HIV has proven an exceptionally difficult foe, says Susan Buchbinder, director of Bridge HIV, a research program at the San Francisco Department of Public Health. “It’s a sloppy virus. When it replicates inside the body, it makes mistakes and doesn’t correct them,” she says. Like photocopies of photocopies, these progeny look ever less like the virus from which they descended. But that variety works to HIV’s advantage. As the immune system targets some viral offspring, others slip through its grasp.
 Yet years of research have revealed clues that may lead to HIV’s undoing. Scientists are learning from the immune systems of rare people, called elite neutralizers, who naturally make antibodies that can stifle many variants of the virus. Researchers had initially assumed these antibodies would target the virus’s protein. But many actually recognize the sugars on its surface. Yet years of research have revealed clues that may lead to HIV’s undoing. Scientists are learning from the immune systems of rare people, called elite neutralizers, who naturally make antibodies that can stifle many variants of the virus. Researchers had initially assumed these antibodies would target the virus’s protein. But many actually recognize the sugars on its surface.
Berman and his team hope to use that insight against HIV. They’re hunting for pieces with the right sugary finish that, in a vaccine, would trigger the same kind of antibodies found in elite patients. That way, a person’s naïve immune system could build its defense before it ever tangled with the virus.
It’s a steep challenge. Berman has pursued an HIV vaccine for nearly 30 years, with failed attempts and inconsistent funding along the way. HIV’s prominence in the public eye declined in developed countries as the virus lost its early, terrifying sting; infection rates have dropped globally and drug therapies make living with HIV manageable. But more than one million people die of AIDS complications each year. A vaccine would finally staunch that loss of life.
Berman has reason to be optimistic. In 2009, a clinical trial showed a vaccine he had helped develop conferred partial protection against the virus. The effect was too small to declare victory, but Berman is building on that success—one sugar-protein fragment at a time—to make a vaccine that can reach the people who need it most. Now, he thinks, he’s close.
Hot pursuit
As a postdoctoral researcher at UC San Francisco in the early 1980s, Berman was at the epicenter when the AIDS epidemic broke. San Francisco in 1981 was ground zero for the initial confusing waves of deaths. “I started seeing people that I interacted with at the university becoming sick and then disappearing,” he says. “It was an eye-opening and scary, scary time.”
 |
| Photo: Carolyn Lagattuta |
| UCSC biomolecular engineer Phil Berman. |
|
Berman started working on vaccines at the biotech company Genentech in the mid-1980s. He was a “gene jockey”: someone who could mix genes from a virus into a mammalian cell. Even though the genes were foreign sets of instructions, the cell would make whatever they prescribed. Engineered cells oozed these virus proteins in 10,000-liter vats towering three stories high. Researchers scooped them up to test them in a vaccine. Like a mugshot, the proteins would give the immune system a chance to learn to recognize its enemy before the fight ever started.
Genentech was initially eager to pursue vaccines, and Berman helped developed one against the virus Hepatitis B. But “it wasn’t a profitable product,” he says. Eventually, the company disbanded its vaccine department in favor of more lucrative drug treatments.
By then, Berman had turned his attention to HIV. Using discretionary time that Genentech encouraged its employees be creative with, he pursued a vaccine anyway. Berman even attracted a handful of other scientists to his "submarine project with low corporate visibility” to find the chinks in HIV’s armor.
HIV is a Russian doll of a pathogen. Its outer sheath is a membrane, an envelope that hides the virus’s inner protein shell and DNA core. For a vaccine candidate, Berman and his team chose a protein called gp120, which sticks out of the envelope like knobby railroad spikes.
The virus depends on these protein spikes. HIV must invade an immune cell to hijack its machinery so the virus can make those imperfect copies of itself. HIV also slips its DNA into the cell’s genome, remaining there as long as the cell lives. But for the virus to get inside the cell, HIV’s gp120 spikes must latch onto its surface. This draws the virus and cell close enough for their membranes to merge. Because the protein is crucial, it’s also vulnerable. A vaccine with a gp120 target primes the immune system to prepare an antibody arsenal.
Antibodies could then stymie the virus. They recognize small areas, called epitopes, of a protein’s bumpy surface. Each unique antibody is like a specialized hand that can only grip one of these spots. By grappling onto an invader, antibodies can block a virus particle from entering a cell.
| Visualization: Samantha Peters |
| This animation depicts the human immune system thwarting the HIV virus. Click on arrow to play. |
|
To test whether their gp120 vaccine could produce these antibodies, Berman and his team turned to primates in 1989. They first injected the vaccine into chimpanzees, then gave them a high dose of HIV. The virus copies itself in chimps but doesn’t cause AIDS. Remarkably, the chimps stayed virus-free.
But the team remained cautious, suspecting HIV could be biding its time. Only after checking the animals for almost a year did the researchers start letting themselves believe their vaccine had a shot at stopping the virus.
“We had to somehow bring it to the attention of the managers that we might have done something good,” Berman says.
Trying times
In 1991, with Genentech’s blessing, the team tested the vaccine in small clinical trials to probe its safety and see whether it induced antibodies. It did. However, the antibodies reacted only against the exact strain of virus upon which the vaccine was based. The vaccine had put blinders on the immune system, giving it a field of vision too narrow to protect against HIV’s many other varieties.
Genentech finally gave up the vaccine when the National Institute of Allergy and Infectious Diseases (NIAID) killed funding in 1994. But Berman and his team’s vaccine fervor was still alive. They raised millions of dollars from private investors and spun off their own company, VaxGen, to refine their product.
Within a few years, the group tested two vaccine versions in large clinical trials. One trial began in 1998 in the U.S. and the Netherlands, another started the next year in Thailand. In 2003, the world learned the results: the vaccines didn’t work. Funding evaporated and VaxGen disbanded. Berman recalls what a shame it was to lose all of the company’s collective expertise and equipment.
 Yet the gp120-based vaccine was down, but far from out. In a Hail-Mary moment, the U.S. Army decided to take VaxGen’s leftover vaccine out of cold storage and test it with a second one in a much larger clinical trial. This second vaccine, made by the pharmaceutical company Sanofi Pasteur, used an engineered canary pox virus to shuttle three different kinds of HIV proteins into the body. Like VaxGen’s vaccine, Sanofi’s had been a dud on its own. Yet the gp120-based vaccine was down, but far from out. In a Hail-Mary moment, the U.S. Army decided to take VaxGen’s leftover vaccine out of cold storage and test it with a second one in a much larger clinical trial. This second vaccine, made by the pharmaceutical company Sanofi Pasteur, used an engineered canary pox virus to shuttle three different kinds of HIV proteins into the body. Like VaxGen’s vaccine, Sanofi’s had been a dud on its own.
The trial, called RV144, took six years. During that time, Berman left industry and moved to UCSC in 2006. “I thought I had done the definitive experiment, the VaxGen trials, and I would never see the vaccine again,” he says.
But three-and-a-half years after volunteers were vaccinated, the combined vaccines had protected 31 percent of them. It was the first—and to date, the only—trial to demonstrate that an HIV vaccine could work at all. Still more tantalizing was its protective effect for volunteers in the first year of receiving the vaccine: a jaw-dropping 60 percent.
“All of a sudden,” Berman says, “this gp120 vaccine was back.”
|
Alison Takemura interviews UCSC graduate student Gabe Byrne about promising steps toward developing an effective vaccine for HIV, the virus that causes AIDS. Click on image to play. |
Error correction
When Berman and his team designed the vaccine, they used the whole gp120 protein to stimulate an immune response. That was a mistake, Berman says. The hulking gp120 structure has dozens of epitopes, spurring the immune system to make antibody matches to all of those sites. Yet the RV144 trial results showed that only a few sites induce antibodies that can actually slow the virus down. Most sites are “decoys,” distracting the immune system from the real targets. It’s one of HIV’s evolutionary tricks.
Another discovery by independent researchers resides in the sugars on gp120’s surface. They make up another layer antibodies can grab onto. But when Berman and his team manufactured the gp120 vaccine 27 years ago, they didn’t know its sugars might matter. So they festooned gp120 with a sugar called sialic acid, which dots the surfaces of our own cells. Its familiar presence helps keep foreign proteins in the body longer.
Adding sialic acid to the HIV protein “was completely the wrong thing to do,” Berman now says.
 HIV’s gp120 bristles with sugars branching in complex patterns like a chaparral thicket. “Antibodies recognize a lot of these unusual carbohydrate structures that we never suspected existed,” Berman says. The sugars, it turned out, are as important as the virus’s protein sequences for protection. HIV’s gp120 bristles with sugars branching in complex patterns like a chaparral thicket. “Antibodies recognize a lot of these unusual carbohydrate structures that we never suspected existed,” Berman says. The sugars, it turned out, are as important as the virus’s protein sequences for protection.
Now, Berman’s group is using the lessons of the RV144 trials to design a better vaccine. The scientists are engineering pieces of the gp120 protein to make targeted antibodies—and then coaxing cells in the lab to add sugars that look more like what the virus displays on its surface.
Choosing just the right fragments lies at the heart of the challenge. Berman is using antibodies from elite neutralizers as bloodhounds to guide his choices.
Making a vaccine
Berman flips open a worn white binder to display a family tree of viruses. All hailed from the same elite neutralizer patient. Red asterisks mark a half dozen of them. These had proven susceptible to so-called broadly neutralizing antibodies, which recognize an unusually broad swath of virus isolates and “neutralize” them, preventing them from entering a cell.
Berman doesn’t just want the viruses that the antibodies recognize now. He wants the strains closer to when HIV first infected the patient. Those invaders, he says, taught the patient’s immune system to produce effective antibodies later on.
But the body lags as a pupil. “It takes two years to make broadly neutralizing antibodies after somebody’s infected,” Berman says. So Berman and his team have reconstructed what an early virus might have looked like. They hope fragments based on these viral ancestors will give the immune system a head start in making these protective antibodies.
Now, the team is screening in rabbits a handful of gp120 pieces as vaccine candidates. Berman is cautiously optimistic. "We're close to picking our target that we want to advance into clinical trials, but we're not quite there yet,” he says.
 |
Infographic: Lindsey Kernodle |
| Click on image to enlarge. |
|
Berman thinks he’ll have a candidate ready for clinical trials in less than two years. To prepare, he’s looking ahead to where the trials might be: southern Africa, he says, where other trials are already under way. His group is redesigning candidates to match the viruses circulating in that part of the world.
"It's one thing to discover something that might work as a vaccine. It's a completely different problem to make it,” Berman says. He attends to both. Can a candidate endure a blistering trek across sub-Saharan Africa? Can a doctor administer it when villages are remote and clinicians scarce? Can it be sold for a dollar or two in developing countries? He’s determined that his team’s vaccine should work under real-world constraints.
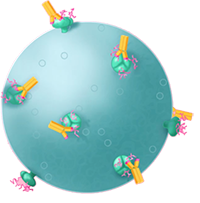 Experts look forward to what Berman emerges with. “Phil was one of the pioneers,” says Larry Corey, president of the Fred Hutchinson Cancer Research Center and a virologist at the NIH-funded HIV Vaccine Trials Network. "His expertise and his knowledge base in how to construct and manufacture these proteins is almost like a lost art.” Experts look forward to what Berman emerges with. “Phil was one of the pioneers,” says Larry Corey, president of the Fred Hutchinson Cancer Research Center and a virologist at the NIH-funded HIV Vaccine Trials Network. "His expertise and his knowledge base in how to construct and manufacture these proteins is almost like a lost art.”
The pendulum of research enthusiasm swung after the initial failed VaxGen trials, Corey says. In the fallout, researchers largely decided that other, non-viral protein vaccines might work better. But the RV144 results and other evidence have sparked a revival in the “gene jockey" approach, he says.
Some scientists dither on whether Berman can make a vaccine in two years. When the virus was discovered in 1984, U.S. Health and Human Services Secretary Margaret Heckler famously missed the mark when a vaccine would be available: two years, she said. But Nancy Haigwood, an immunologist at Oregon Health and Science University and a friendly competitor of Berman’s since the 1980s, says that if he predicts he’ll have a vaccine candidate in two years, he means it. “Phil knows exactly what it takes to get a vaccine into humans,” she says.
Trials ahead
Even if Berman is ready to start testing a vaccine in 2018, he’ll still be climbing uphill. HIV is no longer perceived as the threat it once was. Within the last couple of decades, drug regimens have helped infected people live essentially normal lives. In wealthy countries that can afford to develop a vaccine and bring it to market, that complacency bleeds into public policy. That could pose problems for Berman's team: Clinical trials cost tens of millions of dollars to conduct.
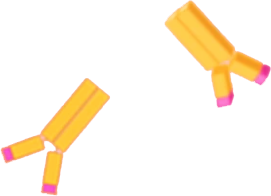 “I do think [the U.S.] Congress is thinking money should be scaled back from HIV—that HIV isn’t such a big problem,” says Buchbinder of the San Francisco Department of Public Health. In 2014, she says, Congress dropped language that set aside 10 percent of NIH’s budget, about $30 billion a year, for HIV/AIDS research. “I do think [the U.S.] Congress is thinking money should be scaled back from HIV—that HIV isn’t such a big problem,” says Buchbinder of the San Francisco Department of Public Health. In 2014, she says, Congress dropped language that set aside 10 percent of NIH’s budget, about $30 billion a year, for HIV/AIDS research.
Yet millions of people worldwide still suffer from the virus, most of whom lack lifesaving treatment. In sub-Saharan Africa, at least 9 million people aren’t taking antiretroviral drugs. Even in the U.S., only four in ten people with HIV are treated. “We have a domestic epidemic,” Buchbinder says.
Despite the funding shifts, director Carl Dieffenbach of the Division of AIDS at the NIAID is adamant: If Berman or someone else makes a promising vaccine, the NIH will find a way to put it into clinical trials. “A safe, effective, durable HIV vaccine is the highest priority of the NIH,” he says. “Period.”
Across the Atlantic, HIV roils South Africa. The country is the current center of the pandemic. About one in five adult citizens—6.3 million people—are infected there, according to a 2014 report by the Joint United Nations Programme on HIV/AIDS.
Poverty, stigma, and rape fuel the spread of the virus, according to Ntando Yolo, an HIV advocate at the Desmond Tutu HIV Foundation in South Africa. Social change is damningly slow. “That’s why a vaccine is really our ultimate hope,” Yolo says.
In South Africa, a followup to the RV144 clinical trial has begun. Clinicians will try delivering the combination of Berman and Sanofi Pasteur’s vaccines more frequently and with different additives to enhance the immune response. These changes could make the vaccines’ protection last longer.
A new vaccine from Berman’s group could be here, too, within a couple of years. Berman’s approach over almost three decades has never diverged from its core: that a mugshot of HIV’s envelope protein can make a person immune. “I think it’ll work eventually,” he says steadily.
In January 2016, researchers estimated that an HIV vaccine would save millions of lives in its first 25 years. Some things are worth the wait.
© 2016 Alison F. Takemura / UC Santa Cruz Science Communication Program
Top
Biographies

Alison F. Takemura
B.S. (biochemistry and cell biology) Rice University
Ph.D. (microbiology) Massachusetts Institute of Technology
Internship: The Scientist
During college, research was a black hole that sucked me in. In that unplumbed darkness, I grappled for footholds to reach my true passion: helping society cope with environmental uncertainty. I tinkered with microbial genomes in a quest for renewable energy, but the light overhead felt eons away. Grounded and more gratifying were my efforts in a group urging MIT to divest from the fossil-fuel economy.
I also loved the radio stories I caught on my walks to lab. The correspondents showed how scientific evidence inspires people: the state climatologist who finally acknowledged the footprints of climate change, the naturalist guide running for mayor in the Galapagos to protect its fragile ecology. With my own stories, I hope to unfurl what scientists know, and move readers’ hearts and minds from apathy into action.
Alison F. Takemura's website
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .

Samantha Peters
B.S. (biology, emphasis neurobiology) University of Texas at Austin
Internships: Smithsonian National Museum of Natural History (Washington D.C.), Lady Bird Johnson Wildflower Center (Austin)
Samantha is a science illustrator based in Texas. After receiving her B.S. from the University of Texas at Austin, she attended the School of Medicine at the University of Texas Health Science Center in San Antonio. She left once she realized illustrating her study guides was more fun than actually studying them. Afterward, while living in New York City, she discovered a love of botanical art through classes at the New York Botanical Garden, and decided to pursue science illustration full time.
Samantha Peters's website
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .

Lindsey Kernodle
B.A. (biology) Washington University in St. Louis
Internship: Golden Gate Audubon Society
Lindsey is a science illustrator based in San Rafael, CA. She is passionate about providing clear visual supplements to complicated science narratives. Throughout her college education in biology and her experiences in teaching that followed, she inevitably found herself pulling up pictures online to illustrate terms or drawing them out herself. She was thrilled to discover the field of science illustration, a wonderful way to combine her interests in nature, creativity, and education. Lately her focus has been expanding to include infographics, woodburnings, interpretive panels, and animation.
Lindsey Kernodle's website
Top |
